...
Their Laboratories
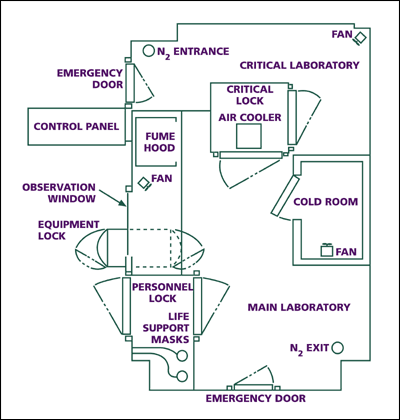
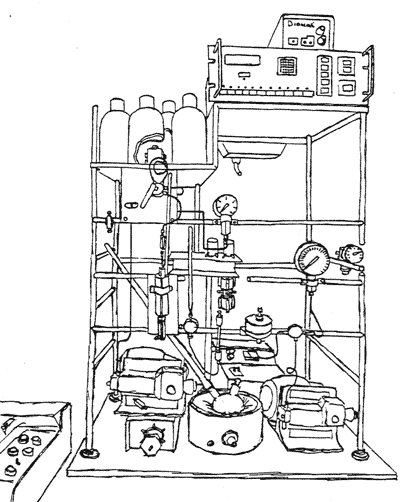
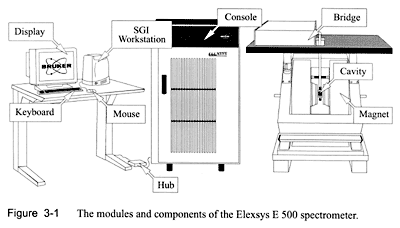
At NIH, Thressa and Earl have worked in the same building, sharing common facilities and instruments. Yet each has maintained her or his laboratory space separately as an independent researcher. A good example of a facility used in common is the anaerobic laboratory in which they have conducted various experiments in oxygen-free conditions. Standard pieces of equipment for biochemical research, such as the centrifuge and the Warburg apparatus, are found in each laboratory. Here is a selected list of facilities and instruments in their laboratories over time.
| Table of Contents | ||||
|---|---|---|---|---|
|
| Div | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
|
...
Running time: 2:10 minutes
Small (56k modem)
Large (ISDN/DSL)
| Div | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
|
| Div | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
|
| Div | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
|
| Div | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
|
| Div | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
|