Tetanus
Tetanus is an acute, often fatal, disease caused by an exotoxin produced by the bacterium Clostridium tetani. Exotoxins are toxins released by a living bacterial cell into its surroundings. Symptoms of tetanus include generalized rigidity and convulsive spasms of skeletal muscles. The muscle stiffness usually begins in the jaw (lockjaw) and neck and then becomes more generalized throughout the body.
Although records from around the 5th century B.C. contain clinical descriptions of tetanus, it was in 1884 when tetanus was first produced in animals by injecting them with pus from a fatal human tetanus case. During that same year, tetanus was produced in animals by injecting them with samples of soil.
In 1889, Kitasato Shibasaburo isolated the organism from a human, showed that it produced disease when injected into animals, and reported that the toxin could be neutralized by specific antibodies. In 1897, Edmond Nocard demonstrated the protective effect of passively transferred antitoxin, and passive immunization in humans was used for treatment and prevention during World War I. A method for inactivating tetanus toxin with formaldehyde was developed in the early 1920s. This led to the development of tetanus toxoid in 1924, which was first widely used during World War II.
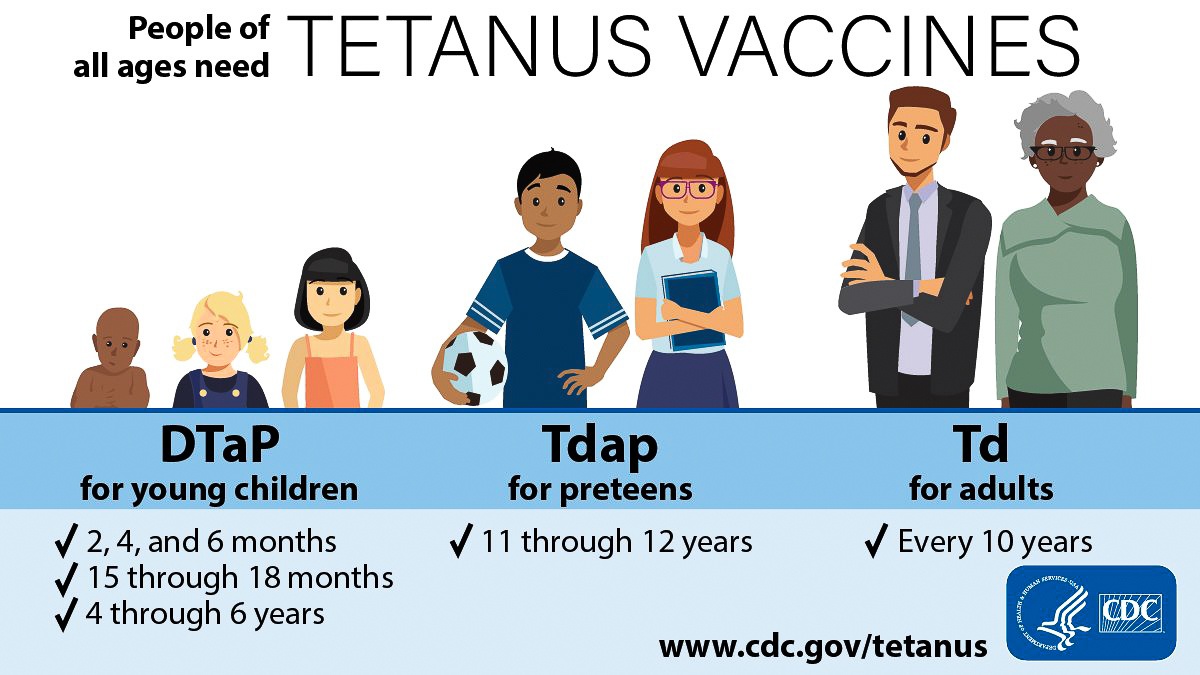
In 1948, the tetanus vaccine became available in a combined vaccine (DTP) with diphtheria toxoid and the pertussis vaccine.
Tetanus toxoid-containing vaccines were introduced into routine childhood vaccination in the late 1940s, making tetanus a nationally notifiable disease. At that time, between 500–600 cases (approximately 0.4 cases per 100,000 population) were reported per year.
After the 1940s, reported tetanus incidence rates declined steadily. Since the mid-1970s, about 50 to 100 cases (approximately 0.05 cases per 100,000) have been reported annually in the United States. More recently, from 2009–2018, an average of 29 (range 18–37) cases were reported per year. Of the 297 cases reported during this 10-year timeframe, there were 19 deaths, all in adults aged 55 years or older. In 2018, 23 tetanus cases were reported, with no deaths.