Hepatitis
Human hepatitis has been recognized since the dawn of recorded history, but proof of infectious cause and delineation of hepatitis A (infectious hepatitis) from hepatitis B (serum hepatitis) were not established until the first half of the 20th century.
Hepatitis A, hepatitis B, and hepatitis C are liver infections caused by three different viruses. Although each can cause similar symptoms, they are spread in different ways and can affect the liver differently. Hepatitis A is usually a short-term infection and does not become chronic. Hepatitis B and hepatitis C can also begin as short-term, acute infections, but in some people, the virus remains in the body, resulting in chronic disease and long-term liver problems. There are vaccines to prevent hepatitis A and hepatitis B; however, there is no vaccine for hepatitis C.
Hepatitis B is a liver disease that can cause mild illness lasting a few weeks, or it can lead to serious, lifelong illness. Hepatitis B is spread when blood, semen, or other body fluid of an infected person enters the body of a person who is not infected.
The first reported case of hepatitis after a blood transfusion was in 1938 by R. Junet and W. Junet in the Revue Médicale Suisse (a medical journal published in French). This became a big issue during World War II when plasma was pooled; one infected donor would infect everyone.
In 1954, the Perlmutter vs. Beth David Hospital case went to court, where a patient sued the hospital where they had been treated for contracting hepatitis after a blood transfusion. The Court of Appeals ruled that the warranties of fitness and merchantability did not apply because the provision of blood by the hospital to the patient was not a sale but part of the patient-hospital contract for services. Blood banks found refuge in early court cases like this one.
In 1964, Grady and Chalmers published an article that blood from volunteer donors carried a lower risk of hepatitis than blood from paid donors. These results were not universally accepted among blood banks. Korzekwa, Jordan, and Alsever published a paper that challenged this and said it was based on socio-economic factors, not payment vs. volunteer donation.
In Kansas City, Missouri medical practitioners decided to boycott blood from the city’s two commercial blood banks. The blood banks sought redress from the Federal Trade Commission (FTC). In 1962, the FTC issued a complaint charging conspiracy to boycott in violation of the FTC Act. The hearing came out in in 1964 in favor of the commercial blood banks. Senate anti-trust hearings were held in 1964 and 1967. In 1968, the civil courts overturned the FTC decision, and no further action was taken on antitrust legislation by Congress. Larger implications of the Kansas City case included that blood was characterized as a commodity in interstate commerce. If that’s true, then blood would be subject to the Uniform Sales Act and the Universal Commercial Code. The Uniform Sales Act and Universal Commercial Code is a comprehensive set of laws governing all commercial transactions in the United States, which helps to increase uniformity in transactions across state lines.
Meanwhile, in 1965, Dr. Baruch Blumberg discovered the hepatitis B virus, winning the Nobel Prize for his discovery. He had been helped by Dr. Harvey Alter at the NIH Clinical Center Blood Bank (and Nobel winner in 2020). Originally the virus was called the “Australia Antigen” because it was named for an Australian aborigine's blood sample that reacted with an antibody in the serum of an American hemophilia patient. Working with Dr. Blumberg, microbiologist Irving Millman helped to develop a blood test for the hepatitis B virus.
1955–1971 was a period of internal strife between blood suppliers and blood users. Dispute between the American Association of Blood Banks (AABB) and Red Cross over replacement policies that left the Red Cross always in debt to an attrition account, a blood debt occasioned by the two for one replacement practices of some hospitals, in the face of the Red Cross policy to replace on a one for one basis.
The year 1971 was a watershed moment: large public frustration that hepatitis was an avoidable sequel to blood transfusion. On November 5, 1971, the NIH Division of Biologics Standards (DBS) acted hastily to publish a requirement that that all blood subject to licensure must be tested for hepatitis B surface antigen. This requirement went into effect on July 1, 1972, the same day that the DBS was transferred administratively from the NIH to the Food and Drug Administration (FDA) Bureau of Biologics. Beginning December 18, 1975, tests of third generation sensitivity for hepatitis B were required for all registered blood establishments.
The hepatitis program was part of the DBS Laboratory of Virology (called the Division of Virology under FDA) with Dr. Lewellys F. Barker until the summer of 1973. In June 1973, with the recognition of the interrelation between the hepatitis program and needed regulatory reform in blood banking, Dr. Barker was called upon to combine these two elements into a cohesive whole under the Blood and Blood Products Division. Going forward, hepatitis research would be integrated into the FDA blood bank regulatory and research effort, as the government’s role in developing a national blood policy took off.
In 1976, the Cunningham vs. McNeal Memorial Hospital case went to court. The court found that blood is a “product,” and it had been “sold” and that the blood was in defective state. The Illinois legislature eventually nullified this claim by determining that strict liability in tort may not be used as a cause of action in transfusion cases. Only negligence claims are considered to offer any resolution of such cases in favor of plaintiffs. No claim (as of 1981) had been filed on the theory that blood is subject to the Uniform Sales Act because it is regulated as a salable product under the biologics provision of the Public Health Services Act.
Hepatitis Research under the FDA
Hepatitis was a major focus of the Bureau of Biologics’ blood regulatory program early on, from checking the proficiency of blood banks to conduct hepatitis tests, to collaborative research on the virus using animal models with NIH's National Institute of Allergy and Infectious Diseases (NIAID) and the Centers for Disease Control and Prevention (CDC), to further study of donor selection criteria.
Following two meetings of its external advisory Panel on Safety and Effectiveness, which considered published and unpublished data on coordinated clinical trials conducted across the nation, the advisors broadly concurred with the Bureau that hepatitis B immune globulin was safe and effective for hepatitis B prophylaxis. Thus, in 1977 the Bureau issued the first license for this product in post-exposure prophylaxis in the event of accidental encounters such as needle sticks, laboratory accidents, and similar situations.
In 1978, and following efforts over several years, FDA finalized regulations to help lessen the likelihood of hepatitis contamination of blood through labeling. Research suggested a significant difference in likelihood of such contamination from paid blood donors rather than volunteers. Henceforth, blood intended for transfusion had to be labeled as either paid or volunteer sourced.
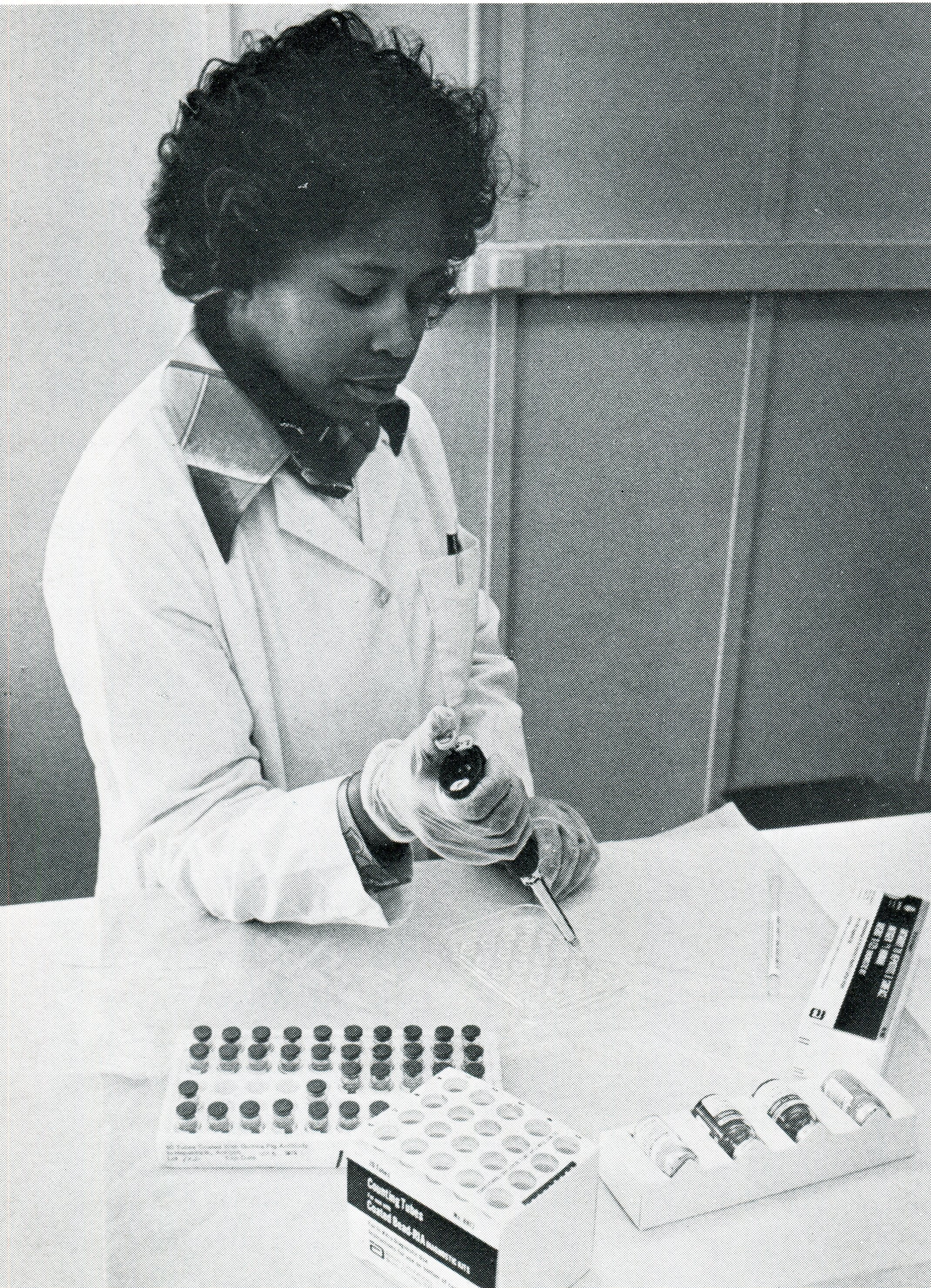
Linda Smallwood of the FDA Bureau of Biologics checks a kit used by blood banks and other facilities to test blood for hepatitis (from FDA Consumer November 1977). FDA History Office
FDA licensed two tests in 1990 to detect non-A, non-B hepatitis. Previously, researchers from CDC and industry constructed a non-A, non-B viral protein from a single stranded ribonucleic acid isolated from an animal model infected with non-A, non-B hepatitis. Recombinant DNA manipulation of this protein produced enough quantity to enable development of a commercial assay to detect antibodies to this viral protein. Clinical trials assessed the ability of this assay to detect antibodies formed against this viral protein, now designated c100-3, in donor blood. The trials showed that eliminating units that tested positive for antibodies to the c100-3 protein could eliminate non-A, non-B hepatitis, and that there was one major non-A, non-B virus, subsequently designated hepatitis C. The first anti-HCV antibody test was licensed in May, and the second two months later.
In July 1986, OBRR licensed the first recombinant viral vaccine, for hepatitis B. The same manufacturer, Merck, Sharp & Dohme, had received a license for a hepatitis B vaccine some years earlier.
In July 1992, the Center for Biologics Evaluation and Research (CBER), which is what biologics regulation is still called at the FDA today, issued the first license for a treatment of chronic hepatitis B for recombinant interferon alfa-2b. FDA had previously approved this for other indications, including hairy cell leukemia. CDC estimated at that time 750,000 to 1 million Americans had the hepatitis B virus, and one-quarter would develop chronic, active hepatitis.
FDA announced in 1990 the first screening test for Hepatitis C (from the Hartford Courant, Friday, May 4, 1990). FDA History Office
Vaccines
Development of the present killed hepatitis A vaccine depended on a series of breakthrough discoveries made during the last half of the 20th century. These were marmoset propagation (1967), definition of virus attributes (1974-1975), development of diagnostic tests and seroepidemiology (1974-1975), and the preparation and proof of efficacy of a prototype killed hepatitis A vaccine (1976). Successful cultivation of hepatitis A virus in cell culture in 1979 quickly led to development of both live and killed hepatitis A vaccines for tests in humans (1980-1990). The year 1991 marks the initiation of protective efficacy trials of two different killed virus vaccines in human beings. The safety and protective efficacy of the first hepatitis A vaccine (manufactured by Merck) was reported on in an article by Maurice Hilleman in 1993.
Also, the NIH's National Institute of Allergy and Infectious Diseases (NIAID) Drs. Robert H. Purcell, Suzanne U. Emerson, and Jeffrey I. Cohen, and the FDA’s Drs. Stephen Feinstone and Richard Daemer of the Center for Biologics Evaluation and Research (CBER), developed and patented a hepatitis A virus (HAV) and related technology used to develop the vaccine. The patents cover a strain of human HAV, HM175, an attenuated form of HM175 and the methods developed to isolate and grow the viruses in cultures of kidney cells derived from African green monkeys. In 1985, based on the scientific and commercial potentials of the NIAID inventions, SmithKline Beecham took a nonexclusive license on the patents and, in 1986, established a cooperative research and development agreement to develop HAVRIX, the world’s first commercially available HAV vaccine. HAVRIX uses an inactivated HM175 strain of HAV. In 1994, SmithKline received the European Prix Gallen award for HAVRIX in honor of its overall contribution to medicine in terms of safety, efficacy, and innovation. Currently, HAVRIX is registered in more than 40 countries.
In 1969, four years after discovering the hepatitis B virus, Drs. Blumberg and Millman developed the first hepatitis B vaccine, which was initially a heat-treated form of the virus. In 1981, the FDA approved a more sophisticated plasma-derived hepatitis B vaccine for human use. This “inactivated” type of vaccine involved the collection of blood from hepatitis B virus-infected (HBsAg-positive) donors. The pooled blood was subjected to multiple steps to inactive the viral particles that included formaldehyde and heat treatment (or “pasteurization”). Merck Pharmaceuticals manufactured this plasma vaccine as "Heptavax," which was the first commercial hepatitis B virus vaccine. The use of this vaccine was discontinued in 1990 and it is no longer available in the U.S.
In 1986, research resulted in a second generation of genetically engineered (or DNA recombinant) hepatitis B vaccines. These new approved vaccines are synthetically prepared and do not contain blood products - it is impossible to get hepatitis B from the new recombinant vaccines that are currently approved in the United States.
In early 1995, FDA issued the first license for a vaccine to prevent hepatitis A in adults and children. The highly contagious disease affected 150,000 Americans annually, usually via contaminated food or water, raw or undercooked shellfish source from contaminated waters, or through a close contact with an infected person.
There is currently no vaccine for hepatitis C.