Building 29A
Rob Tucher Photography
Building 29A
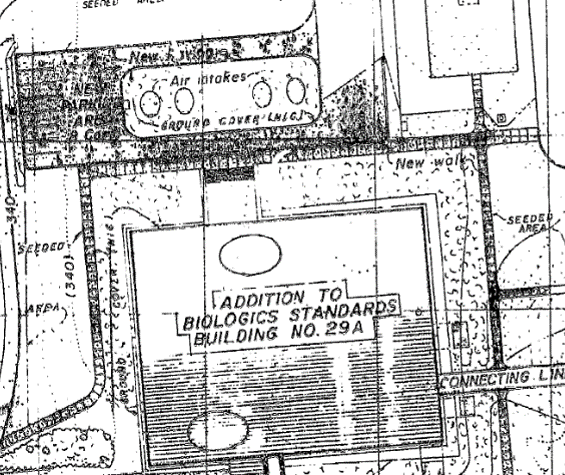
Site Plan of Building 29A showing Air Intake Towers in Parking Lot Median NIH Office of Research Facilities 1964
Building 29A was designed to be an Annex for the Division of Biologics Standards Laboratory and was built from 1965 to 1967. The building does not contain the Georgian Revival style features that are present on the historic core of the NIH campus. Compared to construction drawings, the floor plan of Building 29A has not changed much since it opened in 1967. When the building was vacated in 2014, most of the laboratory equipment was removed, but some interior spaces do still convey the significance of the laboratory function.
Building 29A was always intended as an annex of additional space for Building 29, and the two functioned as a complex for biologics research and regulation. Much like Building 29, Building 29A is lacking in architectural ornament and focuses instead on the functionality and utility of the space. This was common for government and institutional buildings in the early 1960s and reflected the tight budgets and timelines for construction. Building 29A differs from Building 29 in terms of floor plan and in the large amount of glazing on the exterior. Building 29A retains its physical integrity and still conveys its 1960s modern design plan and the laboratory functions for which it is known.
Architect/Engineer:
Smith Hinchman & Grylls Associates (Detroit, MI)
Builder, contractor, suppliers:
Public Building Services of the General Services Administration (GSA) administered the contracts, under the direction of Bernard L. Boutin. George Hyman Construction Co. served as the general contractor. W.G. Cornell Co. was the mechanical subcontractor, with E.C. Ernst, Inc. serving as the electrical subcontractor.
Building 29B
Building 29B was constructed from 1993 to 1994 as additional space for the FDA Center for Biologics Evaluation and Research (CBER). It is connected to Building 29A by pedestrian bridges at three levels and at the basement level.
The five-story, 91,000-square-foot laboratory building includes a basement and penthouse.
Each floor has two wings with offices and laboratory and support space.
The upper three floors have the capacity for specialty Biosafety Level 3 (BSL-3) and/or Curren Good Manufacturing Practice regulations (cGMP) facilities for the production and storage of biological products.
When the FDA CBER left the NIH campus in 2014, Building 29B underwent some renovations and has since been used as overflow space for NICHD, NIAID and NIMHD.
Until 2014, Buildings 29, 29A, and 29B represented the only facilities, not just on the NIH campus but in the entire United States, that have functioned since their construction for the primary purpose of regulating biologics.