Timeline
Pre-1900
1350 BCE
One of the earliest written records of a urine-based pregnancy test can be found in an ancient Egyptian document. A papyrus described a test in which a woman who might be pregnant could urinate on wheat and barley seeds over the course of several days: “If the barley grows, it means a male child. If the wheat grows, it means a female child. If both do not grow, she will not bear at all.” Testing of this theory in 1963 found that 70 percent of the time, the urine of pregnant women did promote growth, while the urine of non-pregnant women and men did not. Scholars have identified this as perhaps the first test to detect a unique substance in the urine of pregnant women, and have speculated that elevated levels of estrogens in pregnant women’s urine may have been the key to its success.
Middle Ages through the Seventeenth Century
Using visual aspects of urine to detect pregnancy became a popular method. In Europe, so-called “piss prophets” claimed to be able to diagnose many different conditions and diseases by the color of urine. In a 1552 text, pregnancy urine was described as: “clear pale lemon color leaning toward off-white, having a cloud on its surface.” Other tests included mixing wine with urine and observing the results. Indeed, alcohol reacts with certain proteins in urine, so this may have had a moderate success rate.
Nineteenth Century
Various theories abounded, such as the possibility that pregnancy urine contained certain identifiable crystals or bacteria. Scientists did not know enough about pregnancy to develop a reliable test. However, for sexually active women, the best method for diagnosing pregnancy remained careful observation of their own physical signs and symptoms (such as morning sickness).
1890s
Many physicians began to describe the workings of chemicals in the body, suggesting that “internal secretions” by certain organs were crucial to an understanding of human biology. Ernest Starling named these chemical messengers “hormones.”
American public health advocates started to encourage women to see their doctors as soon as possible after pregnancy was suspected. Prenatal care was found to improve the health of both infants and mothers, even though most women would not see a doctor or midwife until well into the pregnancy.
1900-1970
1903
Research on human reproduction intensified in the early twentieth century. Ludwig Fraenkel described the corpus luteum, the glandular mass that forms in women’s bodies during the normal menstrual cycle that we now know is supported by hCG during pregnancy. He identified some hormones that had a role in female reproduction, naming the hormone that promoted gestation, progesterone. Progesterone was isolated (an important step in the study of hormones) in 1934.
1920s
Independently, scientists in several laboratories across Europe described the presence of a substance that promotes ovary development and growth in rabbits and mice. In Germany, Selmar Aschheim and Bernhard Zondek noted that this substance specifically affected the formation of the corpus luteum.
Scientists recognized that there is a specific hormone (now known as human chorionic gonadotropin (hCG)) that is only found in pregnant women.
1927
Aschheim and Zondek described a test (known as the A-Z test) which identified the presence of hCG in urine. To test for pregnancy, a woman’s urine was injected into an immature rat or mouse. If the subject was not pregnant, there would be no reaction. In the case of pregnancy, the rat would show an estrous reaction (be in heat) despite its immaturity. This test implied that during pregnancy there was an increased production of the hormone. During early studies of the A-Z test, the scientists discovered that testicular tumors could produce hCG as well.
1930s
Hormone research blossomed in this period. Scientists in several different laboratories developed bioassays (special tests using animals or live tissue) to identify hCG by injecting samples to induce ovulation in rabbits, frogs, toads, and rats. These tests were expensive, required the sacrifice of several animals, and slow, often taking days to get results. The tests were also insensitive when measuring hormone levels to diagnose pregnancy because of the similarity between hCG and another substance, luteinizing hormone (LH). Most bioassays were in fact unable to distinguish between the two except at extraordinarily high rates of hCG.
Herbert Evans discovered that when injected with certain fluids from the female glands a female rat would grow an abnormally large corpus luteum. These fluids were hormones now known as gonadotropins.
In the next few decades laboratory scientists increased their level of interest in the study of human reproduction and on the role of ovaries and testes in human development.
1932
The First International Conference of Standardization of Sex Hormones was held in London, marking the culmination of a decade of increased interest in the chemical properties of sex hormones rather than the previously limited focus on biological function.
1930s-1940s
Popular childbirth books began to encourage women to visit a doctor’s office for confirmation of pregnancy rather than relying on “old wives’ tales” for the diagnosis.
1958
Gonadotropins were first extracted from human pituitary glands.
1960
A “hemagglutination inhibition test” for pregnancy was developed by L. Wide and C.A. Gemzell. Because it used cells in the testing process, this test was an immunoassay rather than a bioassay. The test used purified hCG, mixed with a urine sample and antibodies directed against hCG. In a positive pregnancy test, the red cells clumped, displaying a particular pattern. This test was much faster and cheaper than the old bioassay, but still relatively insensitive, especially for early diagnosis of pregnancy. The test also cross-reacted with various medications.
JV: The problem with these kinds of tests is that there can be some substances in the urine to give a false-negative or a false-positive test at a pretty high frequency, so you had to be careful. It was important to identify what were the interfering substances that gave false results.
Mid-1960s
Important disease research in this period led to more knowledge about how hormones, steroids, and antibodies work in the human body. In the next decade, NICHD scientists would transfer these principles to their studies of reproductive hormones such as hCG.
JV: The first principle of developing a radioimmunoassay was the result of a person making the observation that, when patients with diabetes mellitus were treated with insulin, they developed a circulating antibody. Then after that, we started inducing antibody in animal models, and the rest is history.
JV: There were very few places that were doing reproductive endocrinology research [in the late 1960s and early 1970s] because they didn’t have purified hormones, and there were just some very tedious ways of doing things. To compare the research tools we had back in the late 1970s to now, it’s like Neanderthal to modern man. [There is] no comparison. It took brute force to get some things done.
1966
A. R. Midgley described the first radioimmunoassay for hCG, but the test still could not differentiate between hCG and luteinizing hormone. Several other laboratories reported improvements on this test, but did not solve this basic problem.
1970-2003
1970s
Two things came together in this period along with the so-called sexual revolution: increased research on reproductive health and a heightened desire (brought on by both improved prenatal care and legal abortion) to detect pregnancy as early as possible. Beginning in the 1970s, prenatal care and prenatal testing became more routine in the American health care system. 'A Preliminary screening test for pregnancy,' courtesy of the Food and Drug
1970
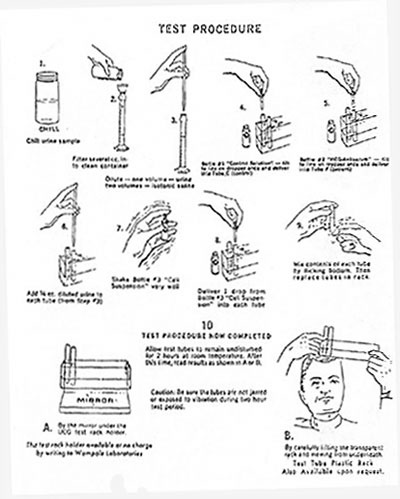
Tests available to doctors and technicians included Wampole’s two-hour pregnancy test. The test could be done as early as four days after a missed period. In the packaging materials, the man pictured performing the test wore a laboratory coat, indicating that it was not intended for home use. Besides the equipment in the kit, (two test tubes, a plastic rack, a bottle of “control solution,” a bottle of “hCG-antiserum” and a bottle of “cell suspension”), testers would need a small funnel and filter paper or centrifuge, clean pipettes or syringes, and saline solution in addition to a urine sample.
"A preliminary screening test for pregnancy," courtesy of the Food and Drug Administration History Office
1970-1972
Scientists at NIH learned more about the properties of hCG. They were specifically interested in which parts of the hormone showed biologic activity. Using various methods, they identified two subunits of hCG and focused on the beta-subunit. They found that the beta-subunit is where the immunologic and biologic specificity of hCG resides (what makes it different from other hormones). Using animal models, they took advantage of this discovery to develop a specific antiserum for measuring the hormone in humans.
In a 1972 textbook on gonadotropins, Vaitukaitis and Ross noted that: “Common antigenic determinants [biological characteristics] among hCG, LH, FSH, and TSH have made the production of specific antisera for radioimmunoassay difficult.” However, the team was close: “the recent isolation and separation of subunits…have provided unique materials with which these questions could be explored.”
1972
Vaitukaitis, Braunstein, and Ross published their paper describing the hCG beta-subunit radioimmunoassay that could finally distinguish between hCG and LH, therefore making it potentially useful as an early test for pregnancy. See Vaitukaitis, J.L., Braunstein, G.D., and Ross, G.T. (1972) “A radioimmunoassay which specifically measures human chorionic gonadotropin in the presence of human luteinizing hormone.” American Journal of Obstetrics and Gynecology, 113, 751-8.
1973
The first edition of Our Bodies, Ourselves, the women’s health manual written by the Boston Women’s Health Collective, noted that available pregnancy tests were most accurate if done two weeks after the missed period. Though the authors insisted that instructions for “collecting and submitting your urine are simple,” modern readers might disagree. “Drink no liquids after dinner the night before,” the text instructed, “then as soon as you awake in the morning collect a urine sample in a clean, dry, soap-free jar and take it to a laboratory.” Another possibility was sending the urine sample to a laboratory in North Carolina, after first writing to request the test kit.
Mid-1970s
Though the test was not yet widely available, NIH scientists spread the word about the new radioimmunoassay. At first, the test was found most useful for clinicians in testing and following patients being treated for hCG-secreting tumors. The sensitive radioimmunoassay could tell the doctors if the chemotherapy treatments had worked.JV: We were doing assays for people all over the place. We felt ethically that we had to because it wasn’t available anyplace else. So we used to give out a lot of antiserums to research labs and show them how to set up the assays.
From Directions and Technical Information on UCG-TEST, 1970, courtesy of Special Collections, Northwestern University Library
1976
FDA approval was sought by Warner-Chilcott for e.p.t, the “Early Pregnancy Test” later known as the “Error Proof Test.” e.p.t would become the first home pregnancy test kit on the market in the United States. The makers of e.p.t worked with the FDA to meet all the requirements of the 1976 Medical Devices Act. (The new regulations divided medical devices into three classes depending on potential for harm and misuse.) Approval was also granted to three other tests that were deemed “Substantially equivalent:” Predictor, ACU-TEST, and Answer.
1976
Several articles in the American Journal of Public Health stated that public health would be better served if the average consumer could purchase a home pregnancy test and use it reliably in her own home.
1977
By the end of 1977, e.p.t was ready for the American market. (Because of requirements for the specific wording on packaging and other last-minute details, there is a lag time between FDA approval and wide availability of most medical devices.) In a “Dear Pharmacist” letter from Warner/Chilcott, drug stores were informed that “the e.p.t consumer advertising campaign has been designed to direct the consumer to their drug store to purchase e.p.t”
1978
e.p.t was advertised in major women’s magazines including: Mademoiselle, McCall’s, Redbook, Family Circle, Ladies’ Home Journal, Good Housekeeping, and Vogue. Advertisements appeared later in the year for Predictor, Answer, and ACU-TEST.
The e.p.t test of 1978 was described to the public in Mademoiselle: “For your $10,” the article notes, “you get pre-measured ingredients consisting of a vial of purified water, a test tube containing, among other things, sheep red blood cells…as well as a medicine dropper and clear plastic support for the test tube, with an angled mirror at the bottom.” The test took two hours, and was more accurate for positive results (97%) than for negative (80%). Advantages, noted Mademoiselle, included “privacy and not having to wait several more weeks for a doctor’s confirmation, which gives you a chance, if pregnant, to start taking care of yourself…or to consider the possibility of early abortion.” (Mademoiselle, April 1978, p. 86)
McCall’s magazine claimed that “physicians we interviewed about the tests endorse the concept.” But the editors cautioned that women who get negative results and who still suspect pregnancy should not wait ten days to take the test again “but should seek medical help as soon as possible.” (McCall’s, March 1978, p. 46)
1979
Taking the test at home, noted a 1979 article in Family Planning Perspectives, both protected the privacy of a woman who might not want her doctor to know she is sexually active and gave women a new opportunity to take an active role in their own health care.
1980s
Research increased and educational campaigns were launched to identify the importance of folic acid in early pregnancy and to warn of the dangers of various environmental hazards and alcohol to a developing fetus.
1990s
Advances in the technology of pregnancy tests included the development of new types of antibodies and the use of enzyme labels in place of radioactive labels.
2003
The next generation of home pregnancy tests was ushered in with FDA approval of Clearblue Easy’s digital pregnancy test. In place of a thin blue line, the indicator screen will now display either “pregnant” or “not pregnant.”
JV: The home pregnancy test is probably the most widely used test besides the hematocrit and hemoglobin [the blood test to measure red blood cells and iron levels that is part of the blood workup done regularly at doctors’ offices].
Advertisement for ACU-TEST, American Journal of Public Health, January 1979.
"Dear Pharmacist" letter from Warner-Chilcott, c. 1977. Courtesy of the National Museum of American History, Smithsonian Institution
Note: Quotations labeled “JV” are from an interview with Judith Vaitukaitis, August 18, 2003. Quotations labeled “GB” are from a telephone conversation with Glenn Braunstein, October 3, 2003.