...
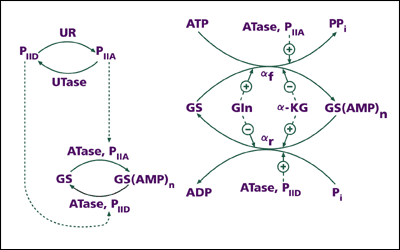
In 1960, Earl went on sabbatical leave to Europe, and this turned out to be a fruitful research experience. Working in Feodor Lynen's laboratory in Munich for half a year, Earl discovered a biochemical reaction dependent upon the vitamin B12—coenzyme. Subsequently, at the Pasteur Institute in Paris, he collaborated with Georges Cohen and others on investigating the regulation of activities of aspartokinase, the enzyme that catalyzes the conversion of aspartate, an amino acid, to its phosphate derivative. At that time it was well known that this conversion was the first common step in a "branched pathway" that led to the biosynthesis of three different amino acids-lysine, threonine, and methionine.
...
| Div | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
|
| Div | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
|