Santiago Ramón y Cajal Exhibit
On March 31, 2014, the second phase of the John Edward Porter Neuroscience Research Center was dedicated. This new facility is shared by scientists from the National Institute of Neurological Disorders and Stroke (NINDS), National Eye Institute (NEI), National institute of Child Health and Human Development (NICHD), National Institute of Dental and Craniofacial Research (NIDCR), National institute of Mental Health (NIMH), National Institute on Deafness and Other Communicable Disorders (NIDCD), and the National Institute of Biomedical Imaging and Bioengineering (NBIB)—and represents a unique opportunity for scientists to collaborate across academic disciplines as well as the boundaries that sometimes separate institutes and centers on the NIH campus.
A new wave of research and exploration is beginning within these walls with new support for the creation of a new arsenal of instruments for unlocking the mysteries of the brain through the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative. This moment may be the most appropriate to look back over the accomplishments of the last century and anticipate those of the next.
This Exhibit currently located in building 35
Ricardo Martínez Murillo, Ph.D., Director of the Instituto Cajal in Madrid, Spain, is pictured in front of NIH's recently dedicated
neuroscience research center where the exhibition of Ramón y Cajal original drawings is located.
Santiago Ramón y Cajal
May 1, 1852 – October 17, 1934
Santiago Ramón y Cajal Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Santiago Ramón y Cajal, a Spanish physician and scientist, was the first to describe the structure of the nervous system with exquisite precision. In what would become known as the “neuron doctrine,” he showed that the nervous system comprises individual cells (later termed “neurons”), that these cells connect to each other at small, specialized contact zones (now known as “synapses”), and that a single nerve cell typically possesses three anatomically distinct structures: the dendritic arbor, the cell body, and the axon. He further posited that neurons function as information processing units, using electrical impulses to communicate within functional networks. Cajal’s experimental work and theories provided the foundation for modern neurobiology.
An exhibition featuring revolving sets of seven original illustrations of famed scientist/artist Santiago Ramón y Cajal (on loan from the Instituto Cajal in Madrid, Spain), may be found near the North Entrance, on the first floor, of Building 35 on the NIH Campus.
Cajal took this photograph of himself in his late-19thcentury laboratory (the shutter controller is cleverly hidden in his right hand). The array of chemicals and dyes he used to prepare tissue slides fill the shelves on the back wall. On his work table sit the microscopes through which he viewed cell structures, the art supplies that he used to render what he saw, and what appears to be a glass of sherry. In this single portrait, we see both the serious scientist and the studio artist. In 1906, Cajal and Camillo Golgi (the Italian physician-scientist who developed the tissue staining technique that Cajal used) shared the Nobel Prize in Physiology or Medicine “in recognition of their work on the structure of the nervous system.”
Each featured original illustration from the early 1900s, is accompanied by a caption written to engage scientists, researchers and investigators who populate the NIH campus. As well as a 3-D printed rendering that enlarges a detail of the illustration above. In this way, the drawings are rendered more accessible to a variety of audiences—including vision-impaired visitors who can directly experience these tactile versions of Cajal's drawings. These files are made available on the 3D Print Exchange. Direct links to the 3-D print files are provided at the end of this page.
The Cajal illustrations currently on-view include:
- Auditory Tracts
- Axonal Tracts in Rat
- Cajal-Astrocytes
- Cajal Astrocytes
- Cerebral Cortex-ii
- Interneuronal Plexuses
- Medulla
Video
5th Installation (current)
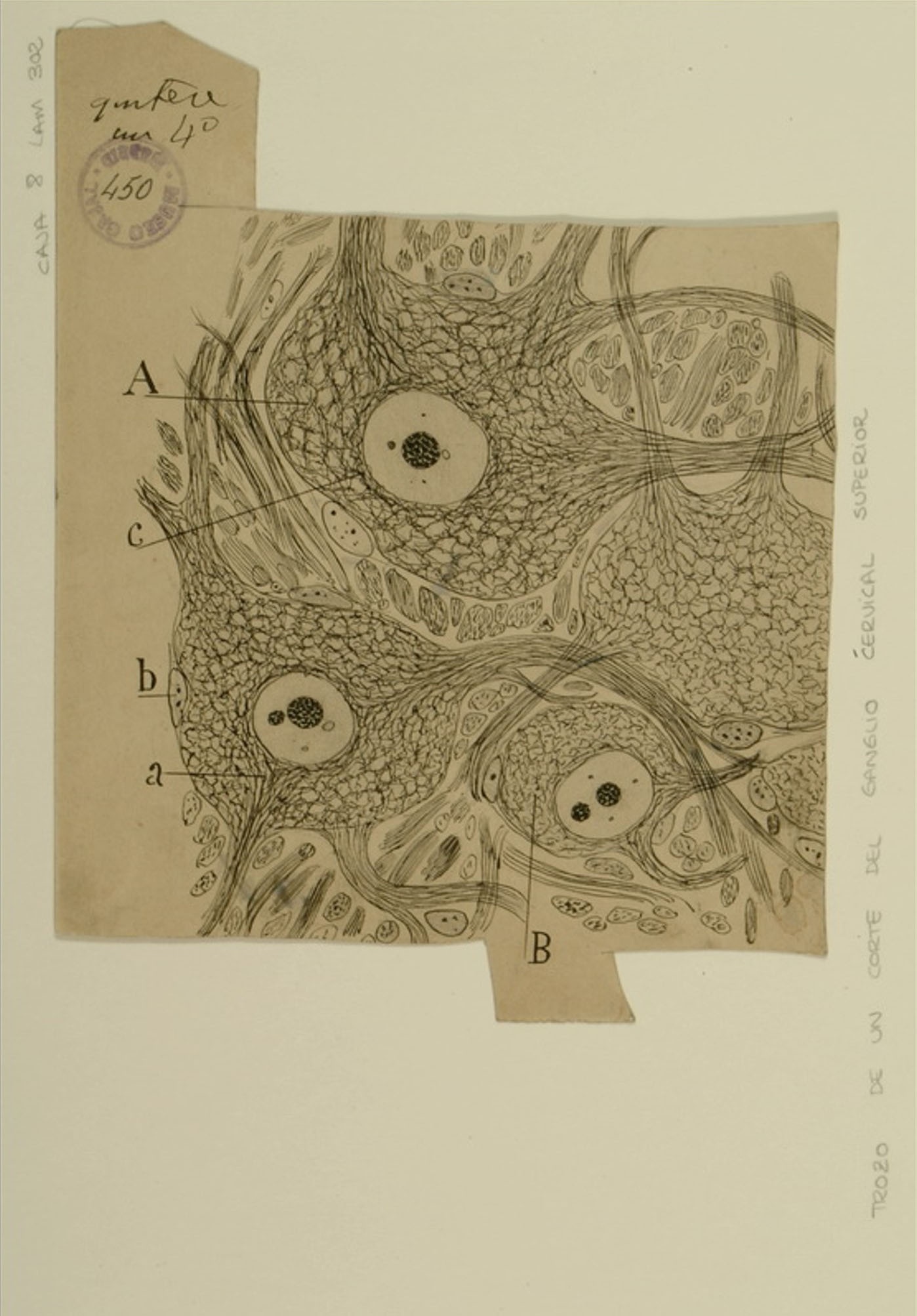
Upper cervical ganglia
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Comparison of visual and olfactory systems
Cajal’s law of dynamic polarization, wherein information flows from the receptive dendrites of a neuron through its cell body and down through its axon (and on through the dendrites of the cells with which it forms synapses), is well illustrated in this figure above comparing the visual and olfactory systems. The olfactory and visual systems were integral to Cajal’s formulation of the law of dynamic polarization, since it was clear where the external stimulus originated and in which direction information ought to flow. Cajal was thus able to hypothesize that the dendrites (or, in photoreceptors, the pigmented outer segments) of the receptor neurons, which were closest to the stimulus, serve a receptive function, and thus, that the axons must be responsible for passing the information onwards. The olfactory system is shown in Fig 1: information originates in the olfactory mucosa (B), where olfactory receptor neurons (a) sense environmental stimuli and send signals to the olfactory bulb (A), which sends it further on to the olfactory cortex (C). This information transfer is unidirectional – signals originating in the olfactory cortex and relayed back to the olfactory bulb travel along a separate set of neurons (e,f,g). Similarly, in the visual system (Fig 2), information travels in the retina from the photoreceptors (a,m), through the bipolar cells (b,n) and ganglion cells (c,o) through the optic nerve to higher visual centers (B). Cajal showed that, in birds, information originating higher in the visual system travels back to the retina along a separate set of neurons (p,q,r).
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
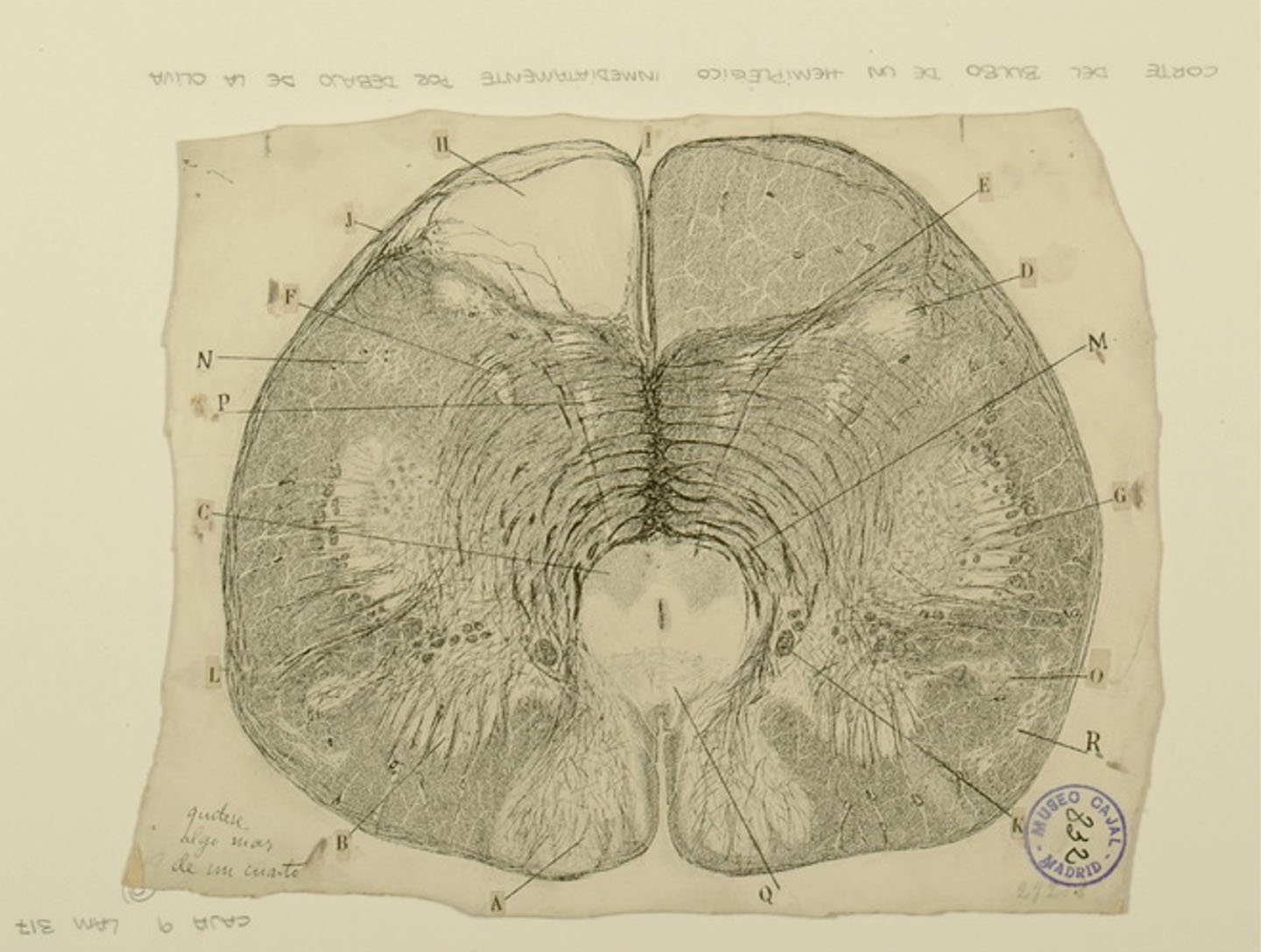
Medulla oblongata from a hemiplegic individual
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
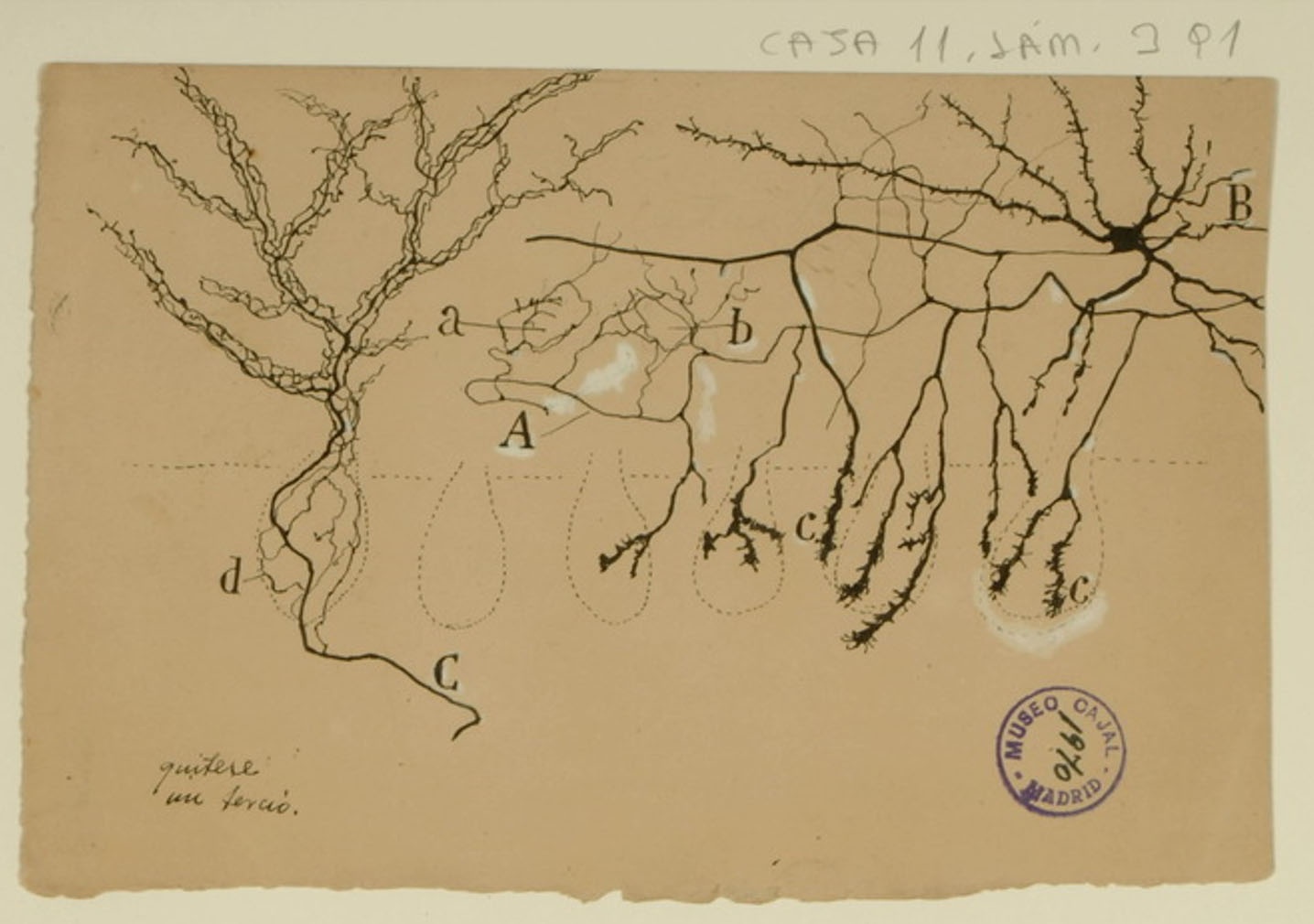
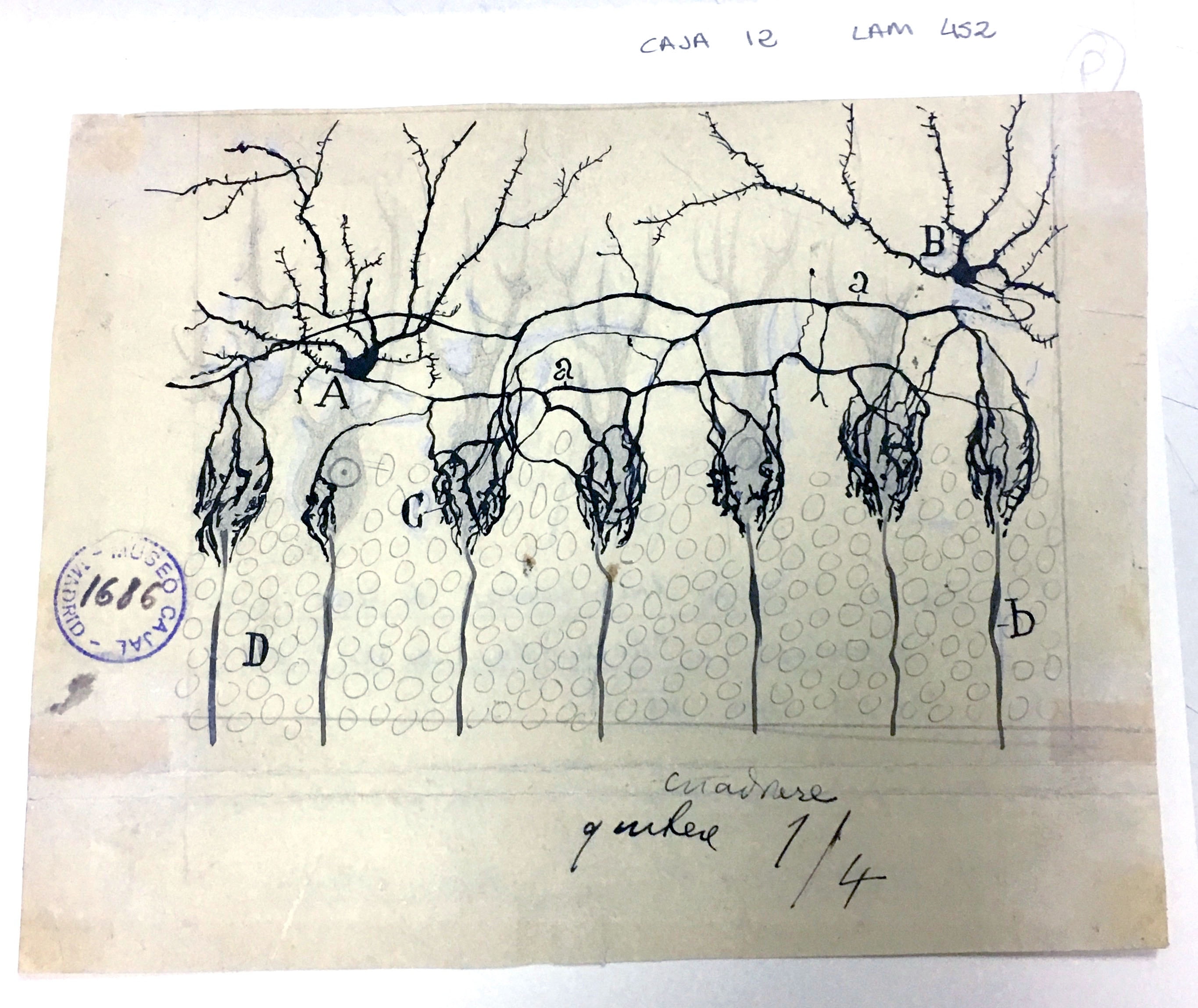
Innervation of Purkinje Cells
Purkinje cells are the main source of cerebellar output, and as such, they must be tightly controlled to correctly regulate motor functions. Basket cells regulate the output of Purkinje cells through the Pinceau formation: fine, brush-like basket cell axons in contact with Purkinje cell bodies (shown above in c; the Purkinje cell bodies are indicated by the faint dotted lines). Cajal described them in 1888, when it was yet unproven that neurons were separate cells rather than an interconnected reticulum, and correctly noted that the basket cells and Purkinje cells were distinct entities. He also correctly noted that the Pinceau formation was an exception to his law of dynamic polarization, where information flows from the axons of one neuron to the dendrites of a neighboring neuron: the axons of both the basket cells and the Purkinje cells interact in the Pinceau formation. He did not, however, foresee the biological importance of the Pinceau formation, which we now know to be critical for normal motor function.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Mouse olfactory bulb
Cajal often used the olfactory system as a subject, finding it to be both easily accessible and regularly structured. Information derived from environmental stimuli that interact directly with olfactory receptors is transmitted to the main olfactory bulb (MOB) or accessory olfactory bulb (AOB) for processing before being transmitted deeper into the olfactory cortex. The MOB (B) receives input from the olfactory sensory neurons in the main olfactory epithelium, while the AOB (A) receives input from the vomeronasal organ via the vomeronasal nerve bundle (D). The AOB is thought to process input mainly from pheromones. Cajal noted that the AOB was composed of 4 layers: the glomerular layer (a), the mitral/tufted layer (b), the lateral olfactory tract (c), and the granular layer (d). Although the MOB and AOB shown above appear to be converging onto a common point, this is not the case – the mitral/tufted cells of the MOB synapse project to the main olfactory cortex, while the AOB mitral/tufted cells bypass the main olfactory cortex and project directly to the medial amygdala and hypothalamic nuclei.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Mammalian retina
Cajal’s fascination with the retina is well documented – his publications on retinal neurons and organization in various species span 45 years, the length of his career. While many of Cajal’s representations of the retina focus on the visual pathway and the directionality of signal flow, this figure depicting a cross section of the mammalian retina showcases the wide variety of cell types therein, particularly amacrine cells (f-n). While the direction of information flow was clear in photoreceptors (ñ and s), bipolar cells (not pictured here) and ganglion cells (o), amacrine cells and horizontal cells (a,b) seemed to defy his law of dynamic polarization. Cajal imagined that the retina transmitted visual information like a complete photograph up into the visual cortex. We now know that the retina actually transmits many photographs in parallel, each depicting specific qualities of the visual world. Horizontal and amacrine cells shape the spatial and temporal characteristics of these parallel images, enabling ganglion cells to encode complex information about color, contrast, motion and other visual features.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
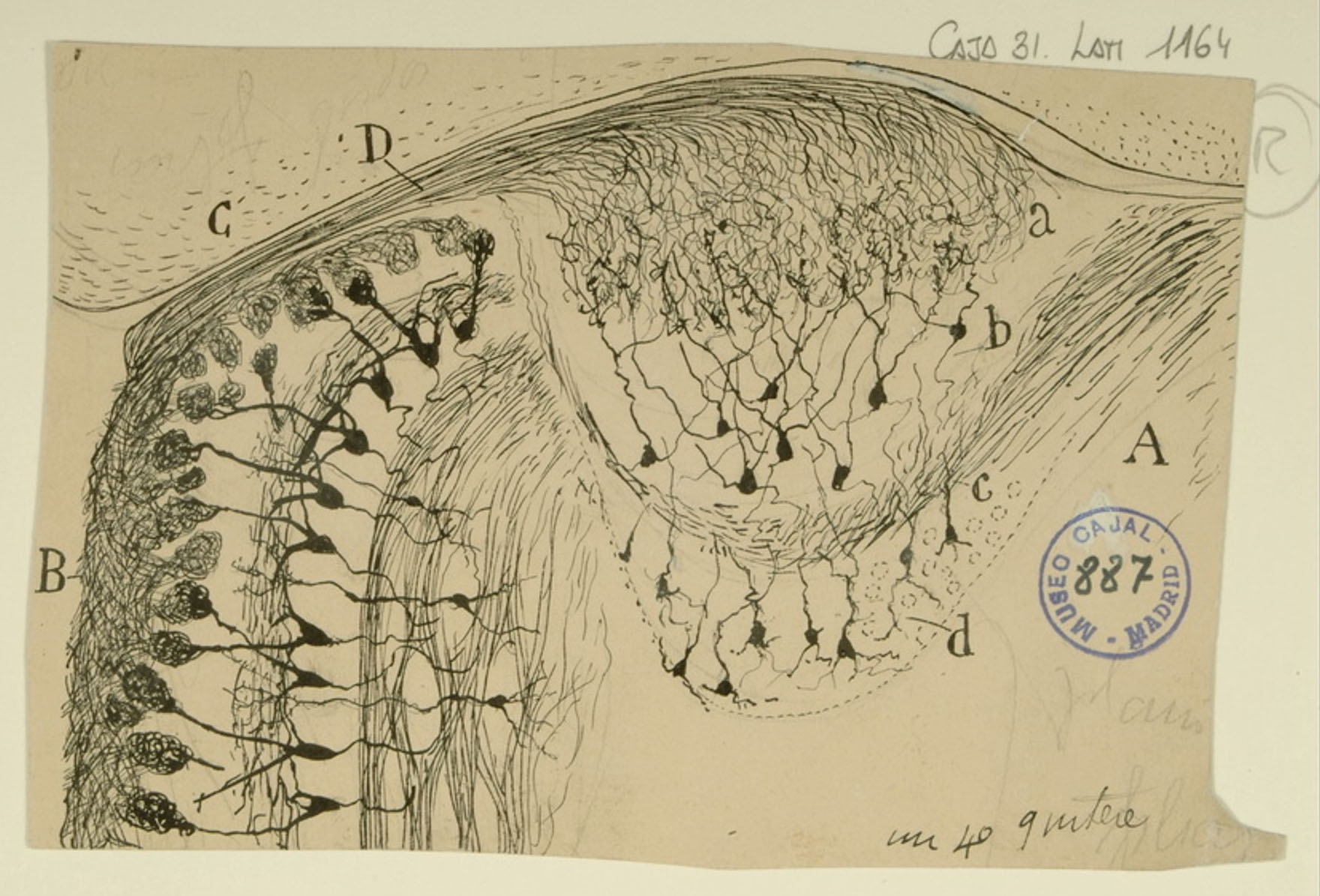
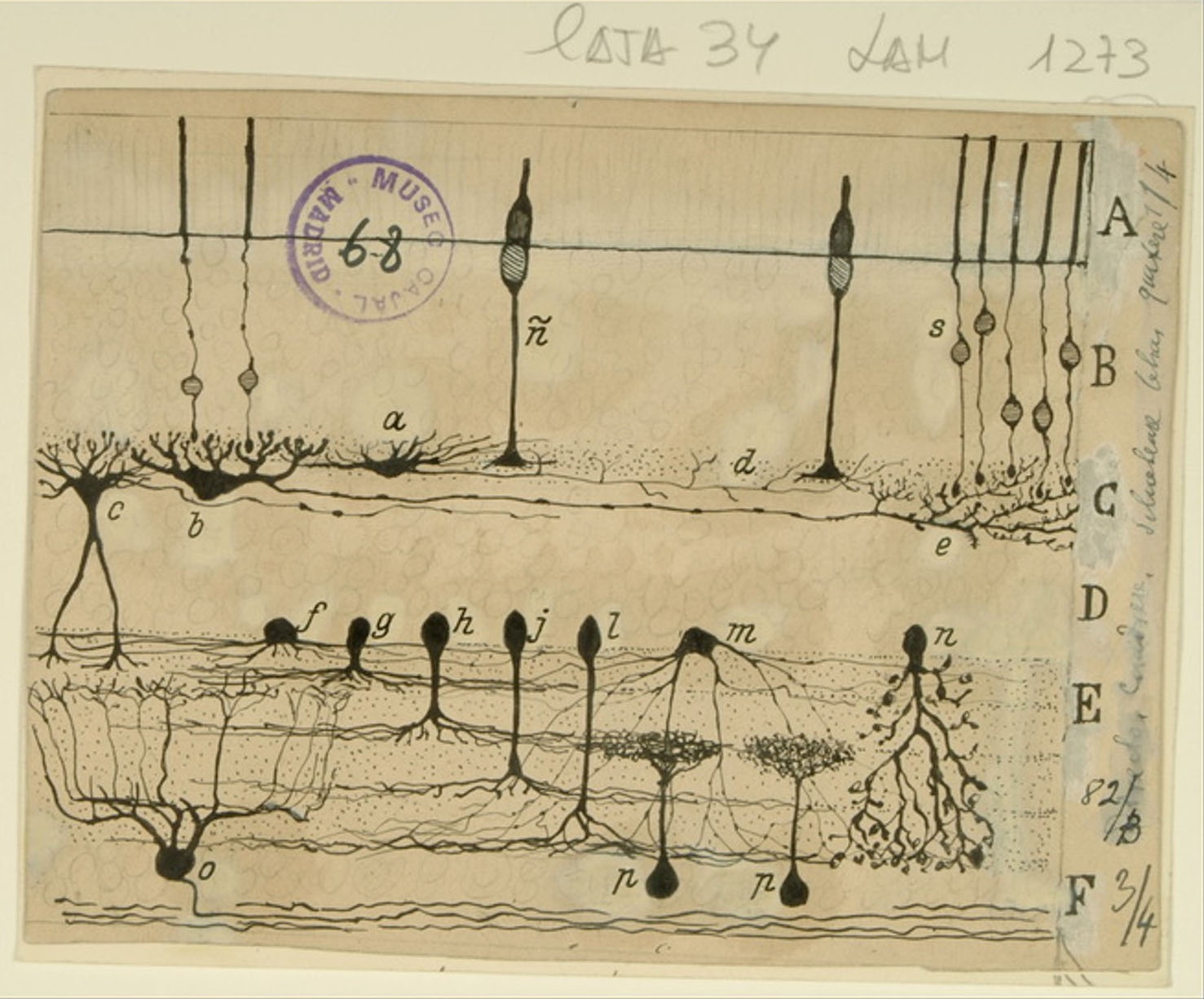
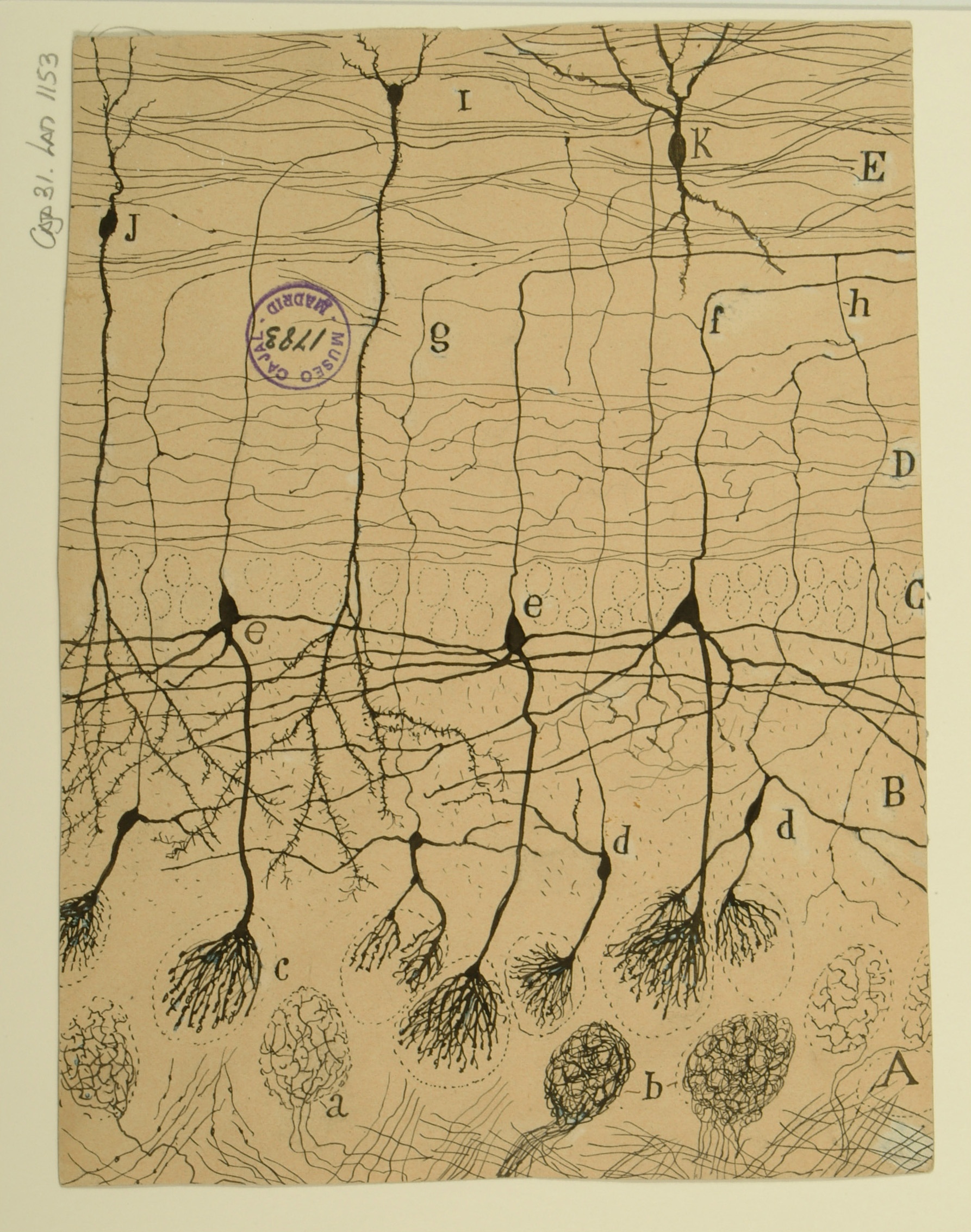
Sphenoidal cortex in a 25-day old infant
The superior temporal gyrus contains the auditory cortex, responsible for processing sound, and Wernicke’s area, which is necessary for the processing of speech to be understood as language rather than simply sounds. Shown here are several layers of pyramidal cells in the superior temporal gyrus, which is layered similarly to other areas of the temporal cortex. Though they vary in size and position, the pyramidal cells (a,b,c,d,e,f,g,h) all exhibit the characteristic cone-shaped cell body, a single apical dendrite extending upwards to the cortical surface, basal dendrites, and basal axons (a). Cajal characterized pyramidal cells from many tissues, detailing the variety of shapes and sizes found in different locations throughout the brain. He also hypothesized that size and shape of the dendritic arborizations of the pyramidal cells would vary over the lifespan of an organism depending; recent research reveals that the cells shown here, from the superior temporal gyrus of an infant, have much larger and denser dendritic branches than those from an adult would in this specific location.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
4th Installation
Nuclei in the auditory pathway
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Modern studies have shown, however, that the inferior colliculus actually processes nearly all the input sent to the medial geniculate body and receives signals from the descending auditory pathway, as well as providing the motor integration necessary for auditory reflexes hypothesized by Cajal, making it a true hub for auditory signaling.
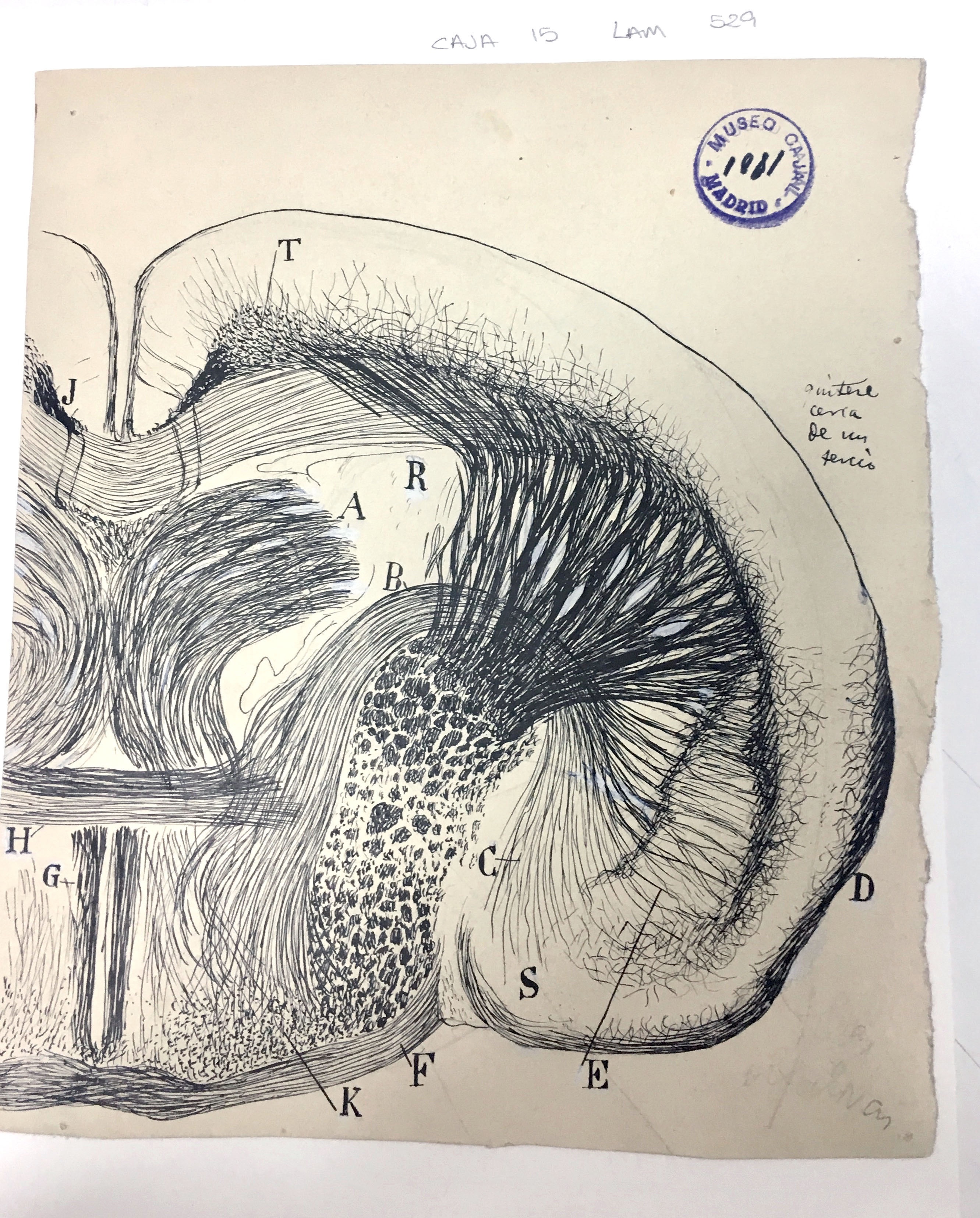
Coronal brain section of a young mouse
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
The lenticular nucleus (E) is a lens-shaped bundle of neurons that, along with the caudate nucleus (R) and the internal capsule, comprises the corpus striatum. Cajal used this drawing in his Texture of the Nervous System of Man and Vertebrates to illustrate the relatively large size of the lenticular nucleus in small mammals – in this case, a mouse – as compared to humans.
Although Cajal posited that the corpus striatum in general was of decreasing evolutionary importance and only useful for the coordination of higher reflexes, we now know that it is important for the facilitation of voluntary movement. The complexity and attention to detail in this drawing showcase Cajal’s skill in translating the view through his microscope lens to the page, where the structures he depicts are easily identifiable to today’s scientists more than 100 years after he put ink to paper.
Basket cells in the cerebellum
Using the silver nitrate staining method to visualize these cells, he recognized that although the axons of the stellate neurons made numerous synapses with the Purkinje neuron cell bodies, they did not fuse at any point. This supported his Neuron Doctrine, wherein the nervous system is composed of distinct cells rather than a network of continuously connected cells, and nervous impulses travel from the axon of one cell to the body of another.
Although he first posited the Neuron Doctrine in 1894, it was not until the 1950s, when the first electron microscopes became available, that scientists were able to confirm the existence of the synapse and thus validate Cajal’s theory.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
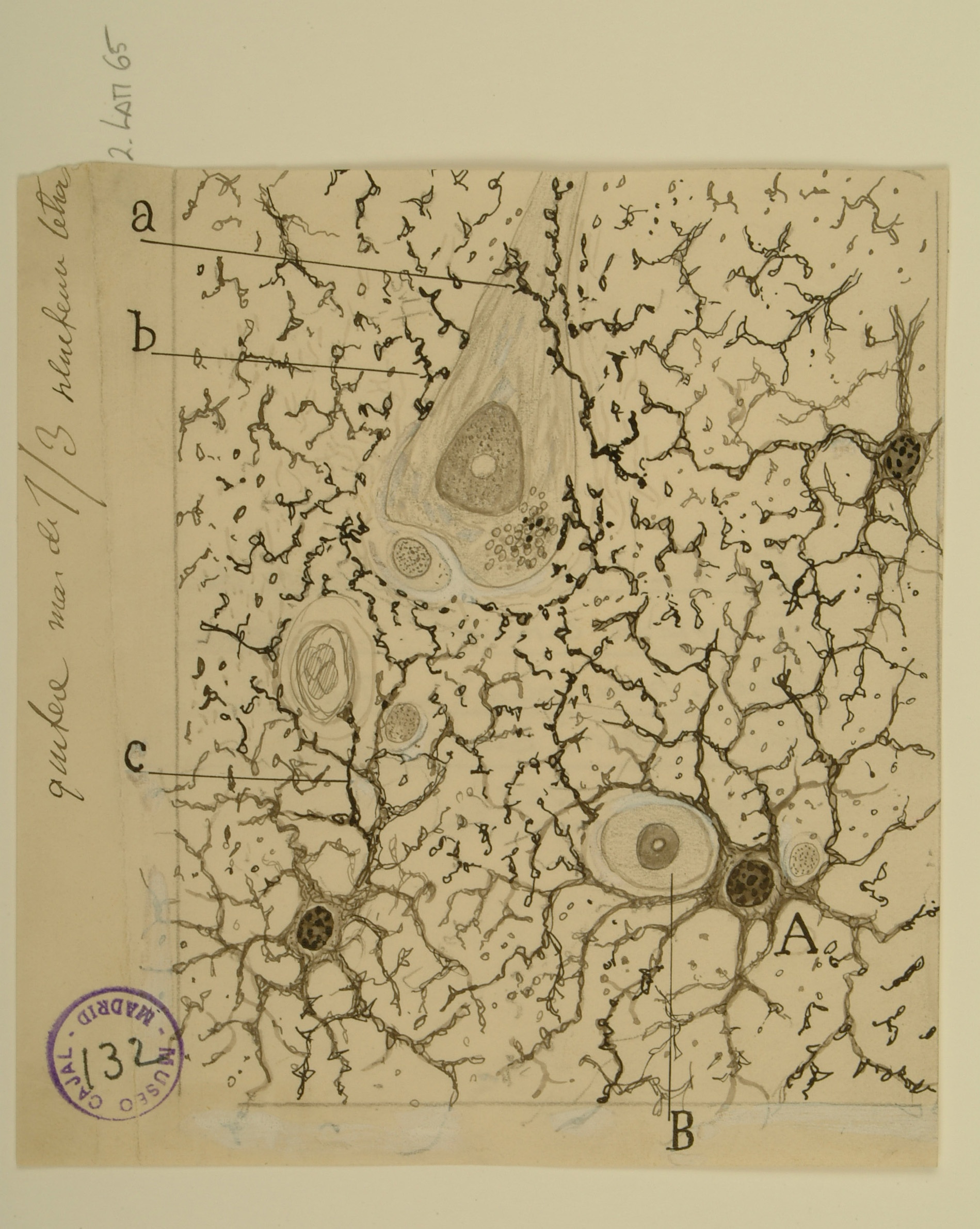
Astrocytes at the border of a wound
Astrocytes are a type of macroglia that are critical for maintaining physiological homeostasis in the CNS and supporting neuronal function. Astrocytes in the grey and white matter of the brain typically have pedicles, or “feet”, that form contacts with capillaries (A, B, e) and control local blood flow.
Using a uranium-nitrate technique specifically for staining astrocytes on a tissue sample bordering a cerebral wound, Cajal observed not only normal astrocytes in contact with capillaries, but also small amoeboid cells (a,b,c). Other scientists, such as Alzheimer, had previously noted such cells in the CNS tissue of persons with various degenerative diseases, but their origins were uncertain. Cajal correctly inferred that these cells were astrocytes which had somehow reshaped themselves after the injury.
We now know that astrocytes become “reactive” after a brain injury: they become polarized, migrate, and their cell bodies swell. Such reactive astrocytes are postulated to have both beneficial (wound healing, limitation of inflammation) and detrimental (scar formation) roles in the response to injury.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Layer 5 of the visual cortex
The gross anatomy and function of the visual system had been a subject of human curiosity for nearly 2000 years by the time Cajal made his careful studies of its constituent neurons in the early 1900s. His meticulous eye allowed him to recognize and distinguish most of the neuronal cell types we recognize today, although he had only the morphology of the stained cells to guide him.
He arranged his descriptions of the primary visual cortex, the first processing center for visual signals within the brain, according to 9 layers clearly delineated by the types of cells present. A subset of these layers are shown above: medium pyramidal cells (B, D, F) in layer 6 give rise to recurrent axons (a) which reach back to the outermost layer of the cortex, while the giant pyramidal cells (A, E) in layer 7 form horizontal dendrite bundles as well as axons that descend into the lower layers of the cortex.
Cajal was the first to realize how short the horizontal axonal/dendritic connections between the cells of the primary visual cortex were, and to hypothesize the significance of this fact: information flows vertically through the layers of the cortex with little lateral spread.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Interneurons of the auditory cortex
While neurogliaform cells do form some classical synapses, of the type that Cajal predicted, it is now known that many of their boutons do not have a specific post-synaptic target; instead, they are able to mediate mass-signal transmission to nearly any neuronal process within their axonal plexus using gamma-Aminobutyric acid. Most modern research on neurogliaform cells has been performed on the hippocampus, where they are hypothesized to provide signaling cues to adult-born neurons.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Cells of the substantia gelatinosa of Rolando
The dorsal horn of the spinal cord contains an area known as both Rexed Lamina II and the substantia gelatinosa of Rolando, named for its gelatinous appearance due to a high concentration of small neurons and a lack of myelination. Cajal classified the cells of the gelatinosa as either “limiting” (b, c) or “central” (d) according to their location within the area, their size, and their dendritic organization; modern neuroanatomists now classify the cells as “stalked” (b,c) or “islet”(d), although there are a variety of neurons in the gelatinosa that defy classification.
Because the axons of many neurons in the gelatinosa appeared not to extend outside of the gelatinosa itself, it was once hypothesized that the gelatinosa was a “closed system;” we now know that neurons of the gelatinosa receive input from the spinothalamic tract and dorsal columns, and relay that information deeper into the spinal cord through Rexed Laminae III and IV.
Neurons of the gelatinosa are unusually dense in Substance P and opioid-type pain receptors, and thus the gelatinosa is believed to play a role in the modulation/mediation of pain perception.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
3rd Installation
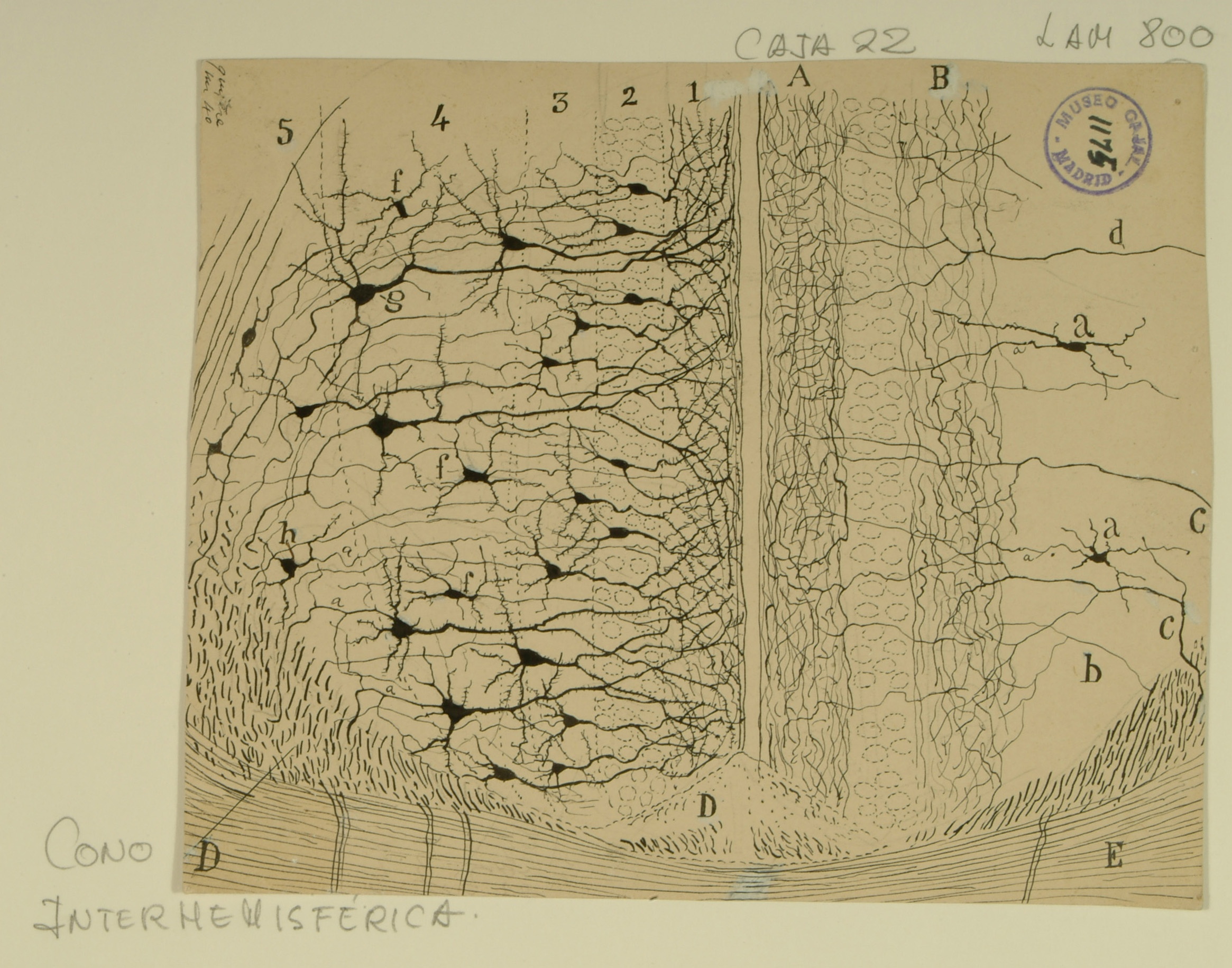
Cingulate Cortex
Among his many “firsts,” Ramon y Cajal was the first to analyze the structure of individual neurons in the cingulate cortex, a structure in the brain involved with emotion formation and processing, learning, and memory. In this drawing, Cajal highlights the structure of the anterior cingulate cortex, as well as its surrounding tissues, the induseum griseum (right D), the cingulum bundle (left D), and the corpus callosum (E).
While the layers of structure are intended to be the same on each side of the drawing, on the left side, Cajal emphasizes the pyramidal neurons present in the five cortical layers of the anterior cingulate cortex (1-5), while on the right side, he draws attention to the interneurons found in those layers (a, d). In addition to the variety of types of pyramidal neurons that comprise the cingulate cortex (extroverted, small, medium, large, fusiform), there are also a variety of non-pyramidal neurons (multipolar, bipolar, and bitufted).
The soma of the neurons and interneurons are mainly in layers 2-5, while layer 1 is made of a dense arbor of dendrites from the layers below. Changes in the number, density, or composition of neurons in the anterior cingulate cortex are associated with disease states such as Parkinson’s disease, Alzheimer’s disease, and schizophrenia.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Diencephalic Nuclei
In this drawing from rat, Cajal shows signals from the interstitial nuclei (c,B) coming from the root of the oculomotor nerve (A), and being sent through the thalamus. But experimental evidence in the past 25 years has revealed that these nuclei actually receive signals from the vestibular nuclei and send signals to the oculomotor nucleus through the posterior commissure (none of which appear in the plane of this illustration), so that information flows in the opposite direction than Cajal’s supposition.
Moving the eye from its resting central position requires sustained movement of the extraocular muscles, but neurons that control various aspects of eye position encode only movement velocity. Consequently, signals must be integrated prior to reaching the oculomotor nucleus, a computation that occurs as they travel through the interstitial nuclei of Cajal.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
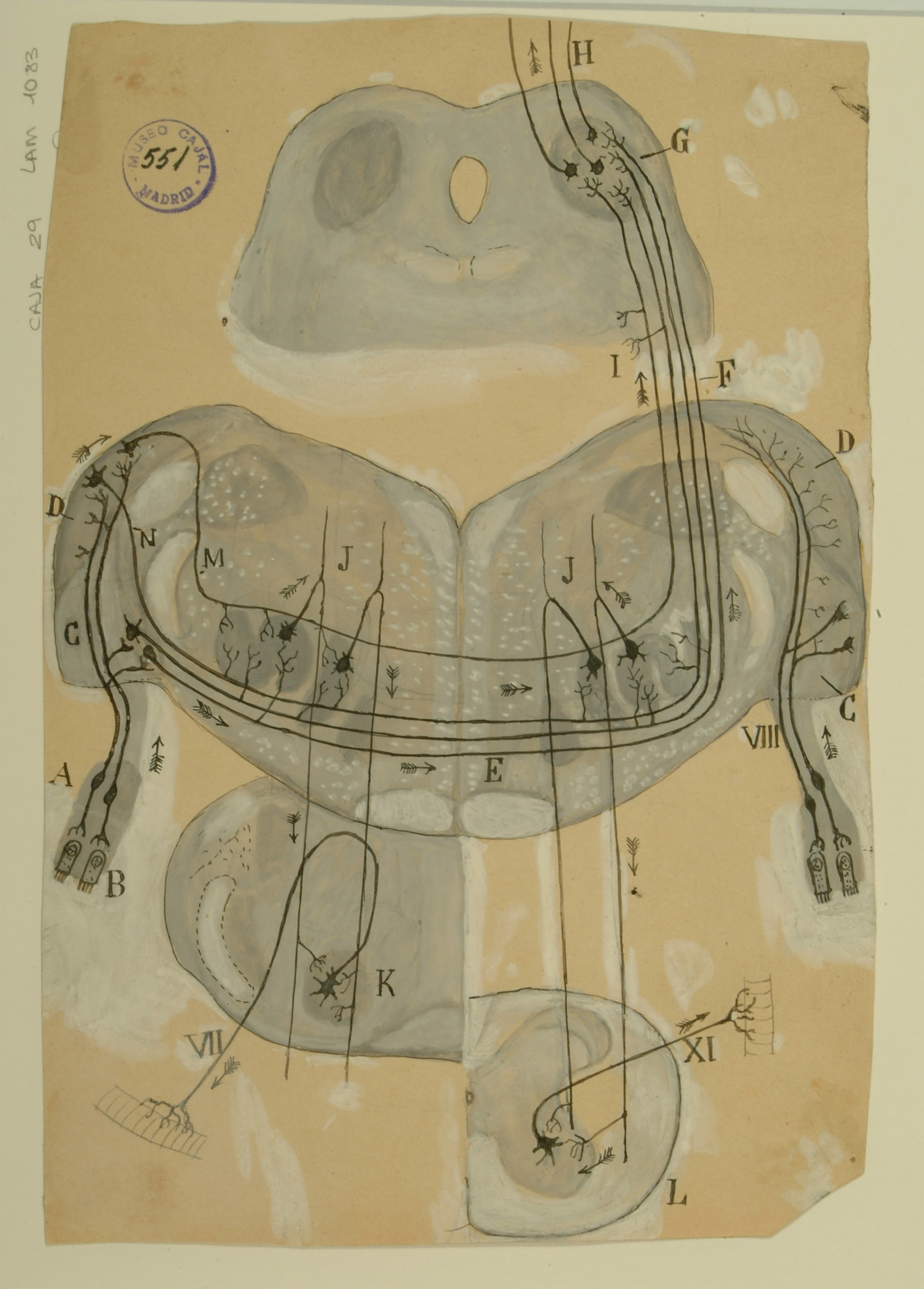
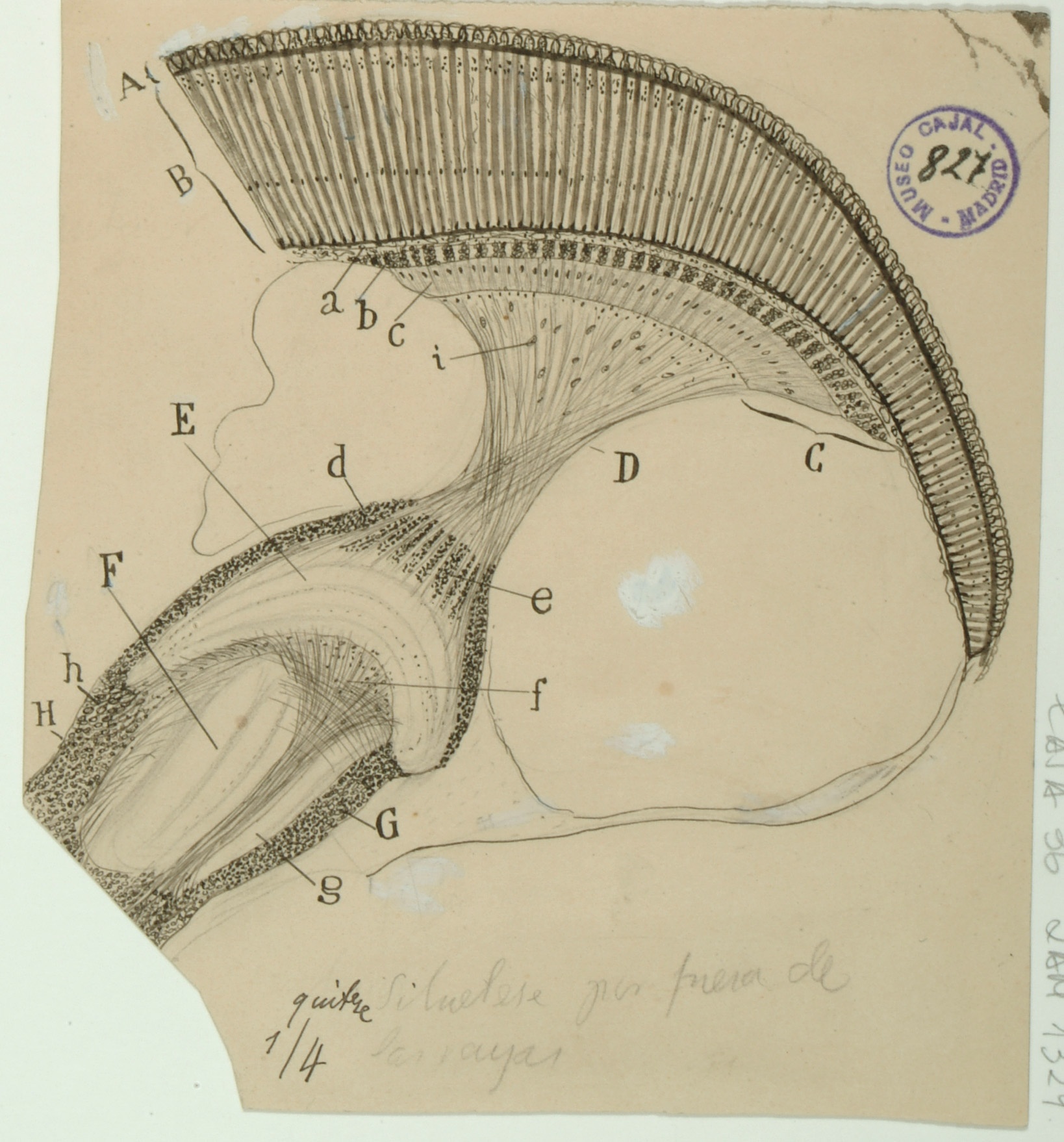
Trapezoid Body
The trapezoid body, located in the brainstem, is part of the auditory pathway where nerve fibers from the cochlea on one side of the brain cross over on their way to the superior olivary nuclei to the other side, which functions in multiple aspects of hearing. This crossing over is believed to help with localization of sound.
Although modern-day diagrams generally show the nerve fibers originating on only one side of the brain for clarity, sections of the trapezoid body examined under a microscope display nerve fibers running in every direction. Despite this, Cajal was able to clearly see the crossing of the nerve fibers from one side of the brain to the other and to correctly infer that signals ran from the ciliated cells in the Organ of Corti in the cochlea (B) up and across through the trapezoid body (E) to the superior olivary nucleus (J) on the opposite side of the brain, and from there up through the inferior colliculus (G) and towards the cerebral cortex.
In addition, Cajal depicts some auditory fibers originating in the superior olivary nucleus as sending signals to the 7th cranial nerve (VII), otherwise known as the facial nerve, as well as to the 11th cranial nerve (XI), which controls muscles in the neck and shoulder. He hypothesized that the connections of these nerves to the auditory system would thus explain the reflex displayed when one hears a sound and immediately turns one’s head and neck in the direction of the source.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
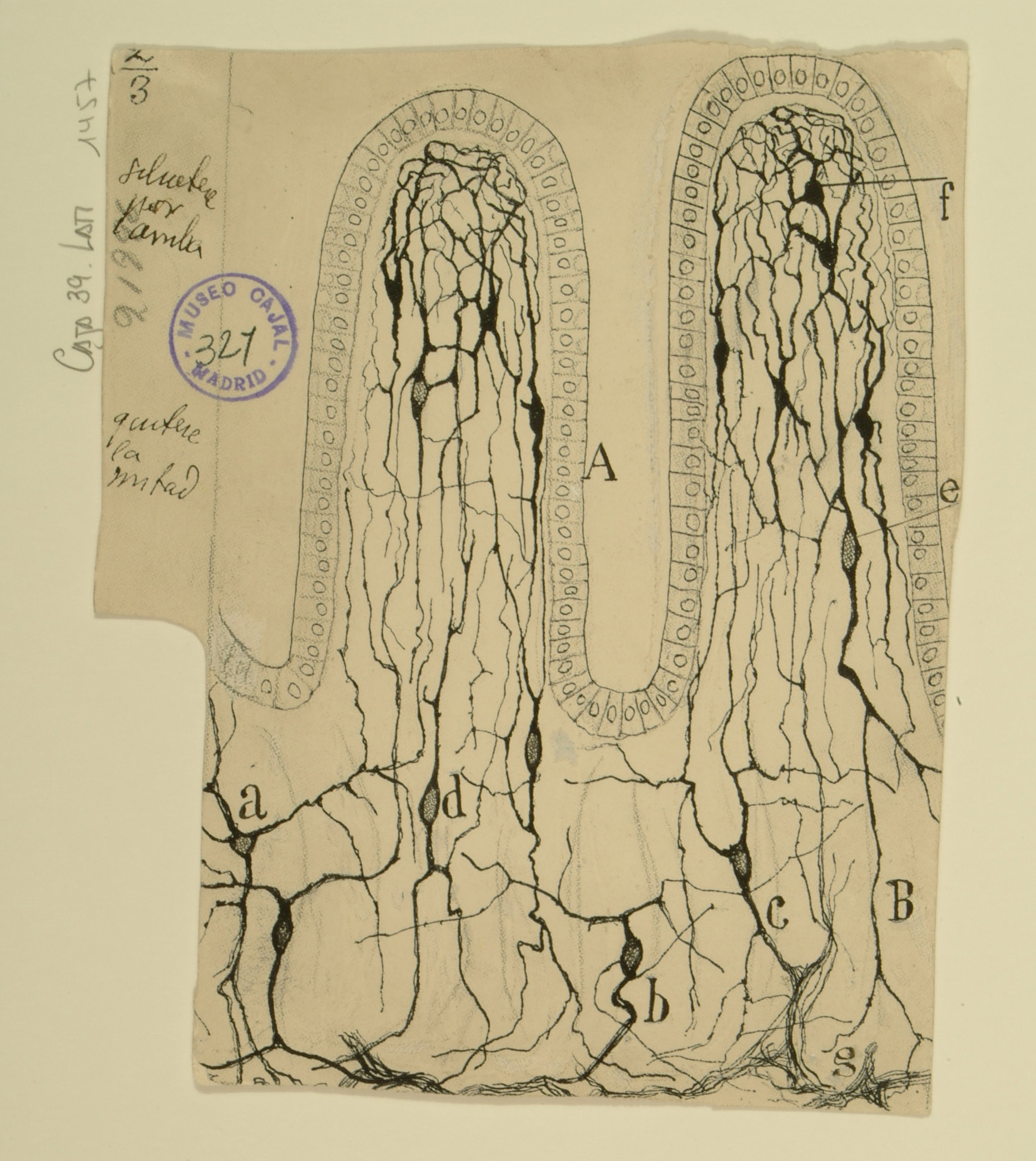
Olfactory Bulb
The olfactory bulb transmits odorant information from nose to brain through six layers of neurons: the olfactory nerve layer (not shown), the glomerular layer (A), the external plexiform layer (B), the mitral cell layer (C), the internal plexiform layer (D), and the granule cell layer (E), which is deepest in towards the brain. Cajal definitively delineated the above six layers in 1890, building on earlier work by Schwalbe, and noted that scent information was not passed from single neuron to single neuron, as in other sensory systems, but was sent from the group of neurons comprising an olfactory fiber to multiple cells in the olfactory bulb.
Cajal was also the first to observe that scent signals are not necessarily transmitted in a serial manner; the mitral cells project into the glomerular layer and are in contact with some ends of the olfactory fibers, and their output is sent to structures deeper within the olfactory cortex. While he identified several types of cells present in the granule cell layer on a morphological and spatial basis, the function of these cells was not elucidated until recently: the provision of inhibitory feedback for mitral cells.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Intestinal Villi
Interstitial Cells of Cajal
Intestinal villi are small projections that extend into the lumen of the small intestine to increase the surface area of the intestinal wall, thereby increasing absorption of nutrients. As the small intestine is made of smooth muscle, one might expect that it is innervated to enable consistent peristalsis.
Cajal’s studies of the innervation of the small intestine revealed several types of cells that responded to the Golgi staining method he usually used. Cajal believed that the stained cells were neurons, but his contemporaries such as Kolliker and Dogiel maintained that the stained cells in the intestinal villi were fibroblasts, as Golgi’s staining method was known to sometimes highlight connective tissue as well as nervous tissue.
This controversy continued until the mid-1990s, when it was determined through the use of marker genes that these cells are generally of mesodermal origin, although some may originate from neural tissue. Such cells are now identified through ultrastructural characteristics and are known definitively to serve as pacemakers for smooth muscle contraction.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Development of Granule Cells in the Cerebellum
The cerebellum was the first system that Cajal studied, and it was through careful observation of its stained tissues that he first became convinced that neurons were distinct cells as opposed to part of a contiguous network, as well as one of the formative systems where he would develop his law of dynamic polarization. The cerebellar granule cell is the most numerous type of neuron in the brain, constituting three-quarters of the neurons therein.
Cerebellar granule cells possess four or five dendrites, each ending in a dendritic claw, a relatively large nucleus, and long, thin axons that split in two to form a “T” shape, forming a structure known as a parallel fiber. Each granule cell gets input from mossy fibers, whose axons fit into the dendritic claws, and sends its output to Purkinje cells through its parallel fibers.
This drawing by Cajal details the development of a cerebellar granule cell from its first appearance as a primary embryonic cell (1), through the beginning of its polar outgrowths (2, 3), formation of a horizontal bipolar cell (4), the start of its descending outgrowth (5, 6), its phase of vertical bipolarity (7, 8), its production of provisional dendrites (9, 10) and, finally, the pruning and refinement of its definitive processes (11, 12). In more recent years, Cajal’s studies of the cerebellum have been corroborated through the use of techniques such as electron microscopy and histochemical staining, which have refined our understanding of the fine organization of the cerebellum.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Fovea Centralis
The fovea centralis, a small avascular depression at the center of the inner retinal surface filled with densely packed cone cells, is responsible for collecting visual information from the focus of visual gaze to form high-resolution images. Signals from the fovea constitute half of all input to the visual cortex.
In this drawing of the foveal pit (F) and perifoveal area by Cajal, the midget bipolar and ganglion cells (so called due to their small size) are highlighted. In the image, each cone cell (A) contacts a single midget bipolar cell (B), which in turn makes synapses with a single midget ganglion cell (C), which then transmits information through the optic nerve to the visual cortex. Cajal correctly noted that as distance from the foveal pit increases, the number of cone cells providing input to a single ganglion cell (C2) increases, a process known as convergence. The lack of convergence at the foveal pit is what allows for maximum visual acuity.
What Cajal did not realize was that, in order to maintain such visual acuity, each cone cell in the fovea is actually connected to two midget bipolar cells and midget ganglion cells (each pair of which exists in mutually exclusive planes of focus in microscope preparations) — one pair will signal only when the center of the cone’s photoreceptive area is stimulated, and the other pair signals only when the off-center area of the cone’s photoreceptive area is stimulated. In addition, information transmitted from the fovea to the visual cortex also contributes to color vision.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
2nd Installation
Growth Cones
The complicated circuitry of the brain and the precise connections of groups of neurons at various places within it suggest that neurons do not randomly form synapses as they grow, but instead respond to organizing signals. Cajal was perhaps the first scientist to observe conical, fluid projections at the ends of developing neurons, and certainly the first to posit that such a structure might be involved in guiding the neuron towards a particular target.
What we now know as growth cones – dynamic extensions of a developing neuron – respond to a variety of chemicals, secreted by target neurons and the extracellular matrix, that can be attractive or repulsive to the growth cone depending on the receptors present.
The variety of the growth cones shown above is due to the complexity of the paths they were to navigate: the ones depicted in C were travelling a quick path through white matter in the brain, while those in A and B travelled a slower, more complicated path through gray matter and the ventral commissure, respectively.
Current research has found that the shape of the growth cone in vivo differs somewhat from Cajal’s preserved specimens, but the complexity of the growth cone in relation to its travel speed remains the same.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Calyx of Held
The Calyx of Held, first described by Hans Held in 1893, is one of the largest synapses found in the mammalian brain. As part of the auditory system, each Calyx is part of the axon of a globular bushy cell in the anteroventral cochlear nucleus, which forms a synapse with a principal cell in the medial nucleus of the trapezoid body. These synapses are integral to detection and localization of high frequency sounds.
At a time when the existence of synapses between nerve cells were not yet accepted as fact, Cajal’s drawings of the mammalian auditory system revealed a sophisticated understanding of the relationship between the Calyx of Held and the neuronal cell body it envelops. Rather than portraying[WC([1] them as a single, connected entity, Cajal’s coloring of this drawing indicates that he knew each part belonged to a separate cell, and that information would need to travel between them.
Because of the large size of a Calyx of Held and its relative accessibility, it has been a popular model system for neurobiological research. In particular, its amenability to patch-clamping has made it a favorite for systematic studies of presynaptic mechanisms, which can be involved in neurological disorders such as Parkinson’s disease.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
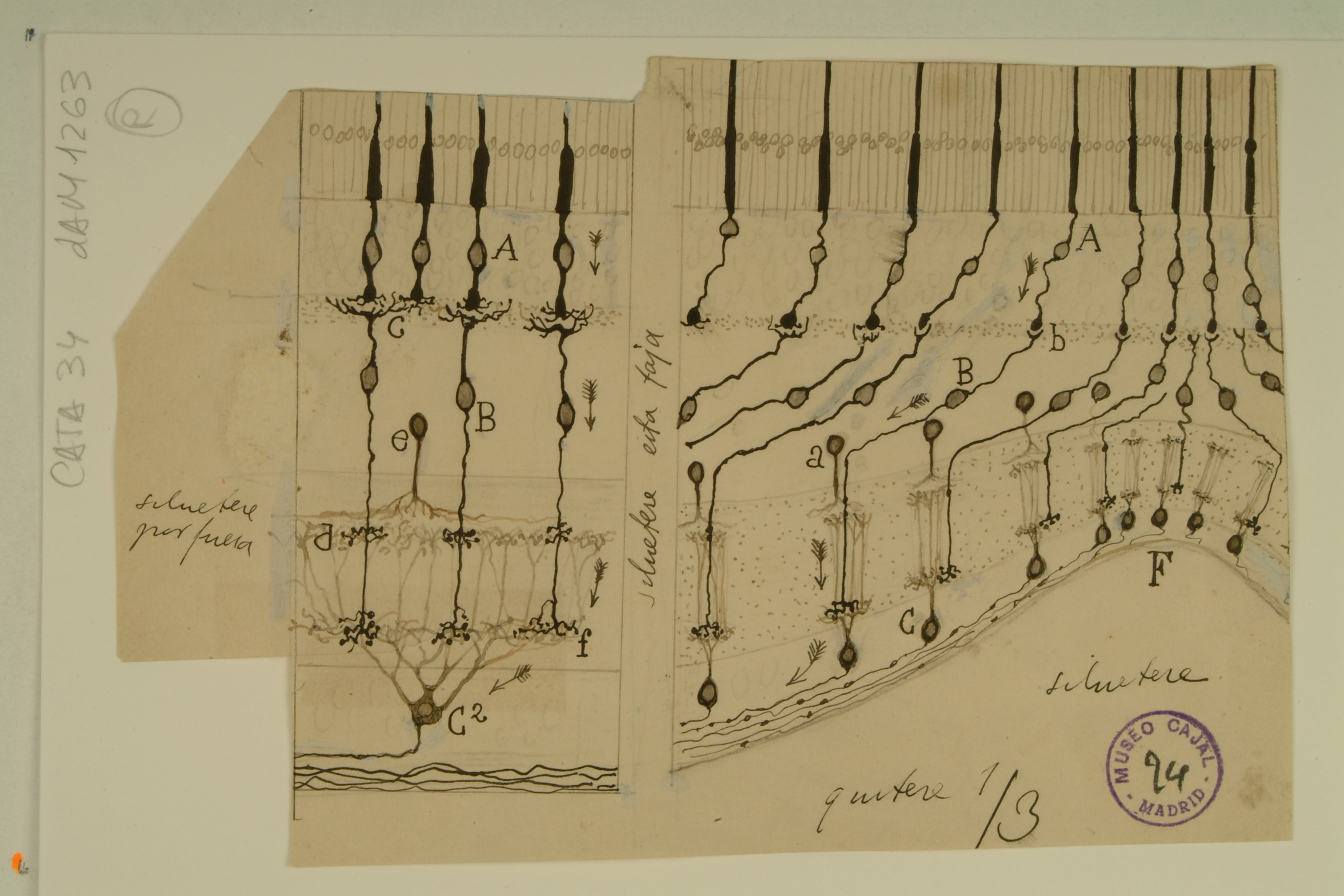
Developing Neocortex
The neocortex, the complex strata of neurons in the mammalian cerebrum, comprises the ridges and grooves seen on the top layer of any 3-D depiction of the human brain. It is involved in processes such as sensory perception, language, and conscious thought.
In the 1890s, Cajal and another scientist, Gustaf Retzius, independently identified bipolar neurons with horizontal nuclei in the developing neocortex of different types of mammals. Shown in layer d of the drawing above, these cells – now known as Cajal-Retzius cells – are not only important for the transmission of information through the neocortex, but they also play a role in brain development. They secrete a protein called Reelin, which is a critical component of a signaling pathway for neuronal migration, ensuring that new cortical cells migrating along the long radial glia (a) and into the upper layers of the neocortex will stop when they reach the correct position.
Diseases associated with a lack of Reelin expression include schizophrenia, bipolar disorder, and autism. Although Cajal was not aware of the existence of Reelin, or the developmental importance of the Cajal-Retzius cells, he accurately captured many of the major features of neocortical development using only his microscope.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Olfactory System
The olfactory system comprises three major parts: the olfactory epithelium, which contains olfactory sensory neurons in direct contact with the environment; the olfactory bulb, which integrates input from the olfactory sensory neurons; and the olfactory cortex, which processes input from the olfactory bulb is processed and transmitts information to other structures in the brain such as the thalamus and hypothalamus.
By observing the positions of the axons and dendrites in relation to each other, Cajal was able to infer the direction of the flow of information in the olfactory system (represented above by arrows) from the periphery of the body to deeper brain structures, nicely illustrating his Law of Dynamic Polarization. Cajal also noted that some information was transmitted back from the cortex to the bulb; he named this bidirectional information flow “centrifugal input”.
The drawing above shows several olfactory sensory neurons (A) forming synapses with a single glomerulus cell in the olfactory bulb (B); in fact, olfactory sensory neurons expressing the same odorant receptor do converge on a pair of glomeruli cells, which integrate the multiple inputs before sending the signal onwards. The olfactory sensory neurons are not necessarily spatially close, but Cajal seems to have found a lucky grouping in this drawing, illustrating again his talent for capturing structures with future significance.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Insect Visual System
Insect eyes are compound eyes: each eye is made of many small unit eyes (ommatidia), as opposed to a single large unit eye as in vertebrates. Cajal’s drawing above details the many ommatidia present in a single fly eye, as well as the neural circuitry relaying visual signals in towards the brain. In the lamina complex, labeled C, we can see the axons of the photoreceptor cells crossing over each other, suggesting neural superposition.
Neural superposition, discovered by Valentino Braitenberg and by Kuno Kirschfeld in 1967, is a phenomenon where photoreceptors from different ommatidia that receive the same signals converge upon the same synapses in the brain, providing vision that is both highly sensitive and high resolution. Cajal was unaware of the phenomenon, but his drawings suggest that he recognized the organization of structures which make it possible.
While visibly different from the vertebrate visual system, the insect visual system shares with it certain similarities in neural circuits: both systems have intermediary neurons that receive information from photoreceptor cells and direct it onwards towards the “neural switchboard” or neuropil.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Information Flow in the Retina
The vertebrate retina is another good example of Cajal’s Law of Dynamic Polarization. Because he knew from which direction
the stimulus to the photoreceptors (at the top of the diagram) originated, it was clear that information ought to be transmitted inwards, through axons of the photoreceptors to the dendrites of the horizontal and ganglion cells, and onward into the visual cortex, as noted by the arrows on the diagram above.
In addition to the usual retinal information flow, Cajal illustrated some more unusual directions for information transmission. He noted information flowing from c to I, along the cell body of a horizontal cell, although this subsequently has not been found to be a viable signal path. Likewise, visual signals in many amacrine cells, shown to the right of D, need not pass through the cell body, contradicting the apparent intent of Cajal’s arrows.
While Cajal may have erred in these two instances, he correctly observed another unusual signaling pathway between G and H: these axons from the visual cortex are returning to the retina and transmitting information to the amacrine cells. This phenomenon has indeed been observed in present day, but not in all vertebrates; significant centrifugal input is a hallmark of avian eyes in particular.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Astrocytes
Once thought to be mere “filler” for the space around neurons, astrocytes are star-shaped glial cells found throughout the brain and spinal cord that are now known to perform many important functions, such as regulating the transmission of ions and glucose between blood vessels and the brain.
When Cajal drew these protoplasmic astrocytes, the prevailing theory was that astrocytes only provided structural support for neurons. He rejected this idea and instead hypothesized that all the astroglia in the brain formed a sort of gland, which would release substances that affect brain function; it is now known that astroglia do indeed release substances that affect neuronal signaling, including glutamate, GABA, and ATP.
Having observed the close association of astrocytes with blood vessels and neurons, as well as the fact that all astrocytes appeared to have a prominent appendage, or “foot,” Cajal further hypothesized that astrocytes used this “foot” to stimulate blood vessel dilation. Astrocyte “feet” do indeed regulate blood vessel diameter, albeit via the release of signaling molecules rather than through physical manipulation, as Cajal imagined. Astrocyte-evoked changes in blood flow, and thus in oxygenation, constitute the signal that is measured by fMRI, a tool used to image brain activity.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
1st Installation
Dentate Gyrus and CA3 Region of the Hippocampus
In this drawing, Cajal details the finer structure of hippocampal dentate granule cell axons, which make mossy fiber synapses onto pyramidal cells in the CA3 region. The relatively narrow focus of this piece allowed Cajal to depict a wealth of detail in both the neurons themselves and the connections between them.
The axons of the granule cells in the dentate gyrus delicately contact the proximal dendrites of the giant pyramidal cells in CA3, while the pyramidal cell axons stretch toward the fimbria, the output of the hippocampus, or send collaterals toward CA1.
Cajal’s major contributions to the field of neuroscience are on display here: the distinct cells, separate yet intertwined, signal the prevailing neuron doctrine, while the arrows signify Cajal’s remarkable intuition regarding the arrangement of the axons and dendrites and the direction of signal transmission according to his law of ‘Dynamic Polarization.’
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Hippocampus
Cajal inferred the flow of information between neurons from their structure and relative position. His ‘Law of Dynamic Polarization’ posits that each neuron is polarized: it has dendrites, through which signals are received, and an axon through which signals are transmitted to the dendrites of the next cells in the pathway. Thus, simply by observing the morphology and location of neurons in a tissue, he was able to discern the direction of signal transmission.
While this was relatively straightforward in the retina, which receives outside stimuli arrive from a particular direction and must be carried inward, Cajal’s real genius was revealed in the deduction of information flow in a tissue such as the hippocampus, where the sites of input and output were not immediately obvious.
The hippocampus, named for its resemblance to a seahorse (genus Hippocampus), comprises the Horns of Ammon (Cornu Ammonis, or CA regions), the dentate gyrus, and the subiculum. It receives signals from various parts of the brain via the entorhinal cortex, and signals flow through the hippocampus in the path shown by Cajal above (dentate gyrus → CA3 → CA1); its output travels through the fornix to the anterior thalamic nuclei and other destinations. Prior to Cajal’s observations, the fornix was thought to be a source of input to the hippocampus.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Spinal Cord
Cajal observed that motor neuron axons originated at the end of the neuron facing away from the brain. Although he did not know the nature of the signal being sent, Cajal hypothesized that the command to move began in the motor cortex of the brain and traveled down the spinal cord to the muscles involved.
In the same way, he noticed that the axons of sensory neurons originated at the end of the neuron that was closer to the brain and inferred that sensory input travels up the spinal cord to be processed by the brain.
These observations were instrumental in the formulation of his ‘Law of Dynamic Polarization,’ which was extended further by Sir Charles Sherrington, winner of the 1932 Nobel Prize in Physiology or Medicine, who showed that spinal cord reflex circuits involved “reciprocal innervation” of opposing muscles.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Continuity vs. Contiguity
Cajal’s greatest contribution to neuroscience is the idea of and supporting evidence for the “neuron doctrine:” the nervous system is composed of individual cells.
Before his precise observations, scientists generally held the reticular theory, championed by Camillo Golgi, who argued that the central nervous system was a continuous network of cells, allowing fast transmission of nerve signals across a large syncytium, and that any observed free nerve endings in the peripheral nervous system were purely receivers of outside stimuli.
Using the silver chromate staining technique pioneered by Golgi, with whom he later shared the Nobel Prize, Cajal observed that cells in the vertebrate central nervous system had free endings that were often apposed to many different neuronal cell types, suggesting that each nerve cell was a separate entity.
In the diagram shown here, Cajal compared Golgi’s theory of continuity (I, left) with the contiguity that he observed under themicroscope (II, right). Cajal’s neuron doctrine was widely accepted after he published his findings in the late 1880s. It was not until the 1950s that electron microscopy by Palade and Palay definitively demonstrated the existence of synapses and the physical separation between neurons.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Cerebellum
In the 1960s, Sir John Eccles used electro-physiological analyses to reveal elegant feedforward circuitry. Incoming mossy fibers deliver sensory signals from pre-cerebellar nuclei and the spinal cord to granule cells (g), the most numerous neuron type in the brain. Granule cell axons bifurcate to form the densely arrayed parallel fibers, which contact the (relatively) giant Purkinje cells (a).
Meanwhile, climbing fibers transmit signals from the inferior olivary nucleus to Purkinje cells. Each parallel fiber makes a single weak synapse onto a Purkinje cell, whereas the parallel fiber connection is very strong, comprising hundreds of distinct synaptic contacts.
Purkinje cells transmit inhibitory signals to the deep nuclei, outside the cerebellar cortex, which will relay information to the cerebral cortex. The cerebellum coordinates and fine-tunes movements, although recent research has revealed roles for the cerebellum in spatial cognition and language.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Retina
Cajal’s careful studies of retinal sections from a variety of animals led him to conclude that retinal structure is remarkably similar between species: all vertebrates have rod and cone photoreceptors, as well as bipolar cells, horizontal cells, amacrine cells and ganglion cells, in roughly the same arrangement.
The vertebrate retina develops as an outgrowth of the brain, and signals received from stimulation by light travel through the retinal network and then into the brain via the ganglion cell axons in the optic nerve. The clear signaling pathway through the retina, from the photoreceptors through the ganglion cells, was one of Cajal’s initial inspirations for his ‘dynamic polarization’ theory.
Further studies of the retina led to the Nobel Prize in Medicine in 1967 for Granit, Hartline, and Wald, for their respective discoveries of the electrophysiological properties of retinal neurons, lateral inhibition among neurons, and the mechanism of rhodopsin function.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Cortical Pyramidal Cells
Neuron theory was advanced through tireless promotion of Cajal’s stained brain sections, in which distinct neuron boundaries were clearly visible. Cajal was able to detect the delicate structure of neurons by applying Golgi’s silver nitrate staining method to samples from embryological or perinatal tissue, in which the neurons were unmyelinated and thus more easily susceptible to staining.
This section of the cerebral cortex from a human infant is an excellent example of the success of his technique. Though their processes overlap, the cells are clearly distinct from one another. By noting the placement of the axons and dendrites, Cajal was also able to postulate the direction in which information flows through this tissue—from the deeper layers of the cortex up toward the surface.
The precentral gyrus is now known to be part of the primary motor cortex, which coordinates with several other parts of the brain to plan and execute muscle movements.
Courtesy of the Cajal Institute, Spanish National Research Council or CSIC©
Test Print
This initial print file was created to create a process for making a 3d printable file from the image data. Two things became apparent in the creation of the file. One is that the relief, the distance between the background (generally paper without ink) and the foreground, neurons and structures inked on the paper, needed to be minimal in order to more effectively communicate the subject through touching with fingertips. If the relief was too high, as in this test print, then it became harder to feel small details.
Creating the 3D objects
Most of the 3D files were created by a process called "Height Displacement", in which the generally lighter background (absent of dark ink) represented the base, and the darker color would be raised "higher" from the background. Several other techniques were used in conjunction or instead of height displacement, including processing with software including Adobe Photoshop and Illustrator, 3D Coat, Blender, and Netfabb.
Close up of the 3D print
Photograph of the 3D printed plates being bonded together with acetone
Acknowledgements
This exhibition is a collaborative effort of the Office of NIH History’s Stetten Museum and the National Institute of Neurological Disorders and Stroke (NINDS). Jeffrey S. Diamond, Ph.D., Senior Investigator, Synaptic Physiology Section, Division of Intramural Research, NINDS, represented the NIH in negotiating the loan of the original drawings from the Cajal Institute of Madrid, Spain. NIH and the exhibition team are particularly grateful to Dr. Juan De Carlos, of the Cajal Institute for making this exhibition possible through the loan of the original drawings, and the archival images featured in the exhibition.
The exhibition team also thanks the National Library of Medicine, History of Medicine Division’s Paper Conservator, Holly Herro, for advising on appropriate exhibition light levels and mitigation strategies. Content and environmental design were created by Hank Grasso of the NIH Stetten Museum. Jamie Kugler, Ph.D., researched and composed the labels identifying for each of the four sets of (seven) drawings. This installation was produced under Chris Wanjek, OIR Director of Communications, Story Landis, Ph.D., (former) Director, NINDS, and Walter J. Koroshetz, M.D., (current Director, NINDS).