Plasma Cell Tumor Development: A Complex Multi-Step Process
What Causes Cancer?
Potter now knew a great deal about immunoglobulins. He knew that they are antibodies that bind to antigens. He knew each one attaches to only one kind of antigen. He knew their three-dimensional structure and basic genetic composition. But there was one question that he still could not answer: Why were the BALB/cAn mice the only ones that developed plasma cell tumors when injected with mineral oil or plastic shavings? In other words, what caused the tumors to develop? The answer would open new fields in cancer and immunology.
“He tirelessly examined tens of thousands of stained tumor slides. Towers of stacked slideboxes teetered around him and occasionally came crashing down.”
- —Gary Jones, NCI
Olympus Compound Microscope
National Library of Medicine. Learn more about Potter's Olympus Microscope (TODO: Link)
Permanent collection of the NIH Stetten Museum. Courtesy of Dr. Beverly Mock, NCI National Library of Medicine
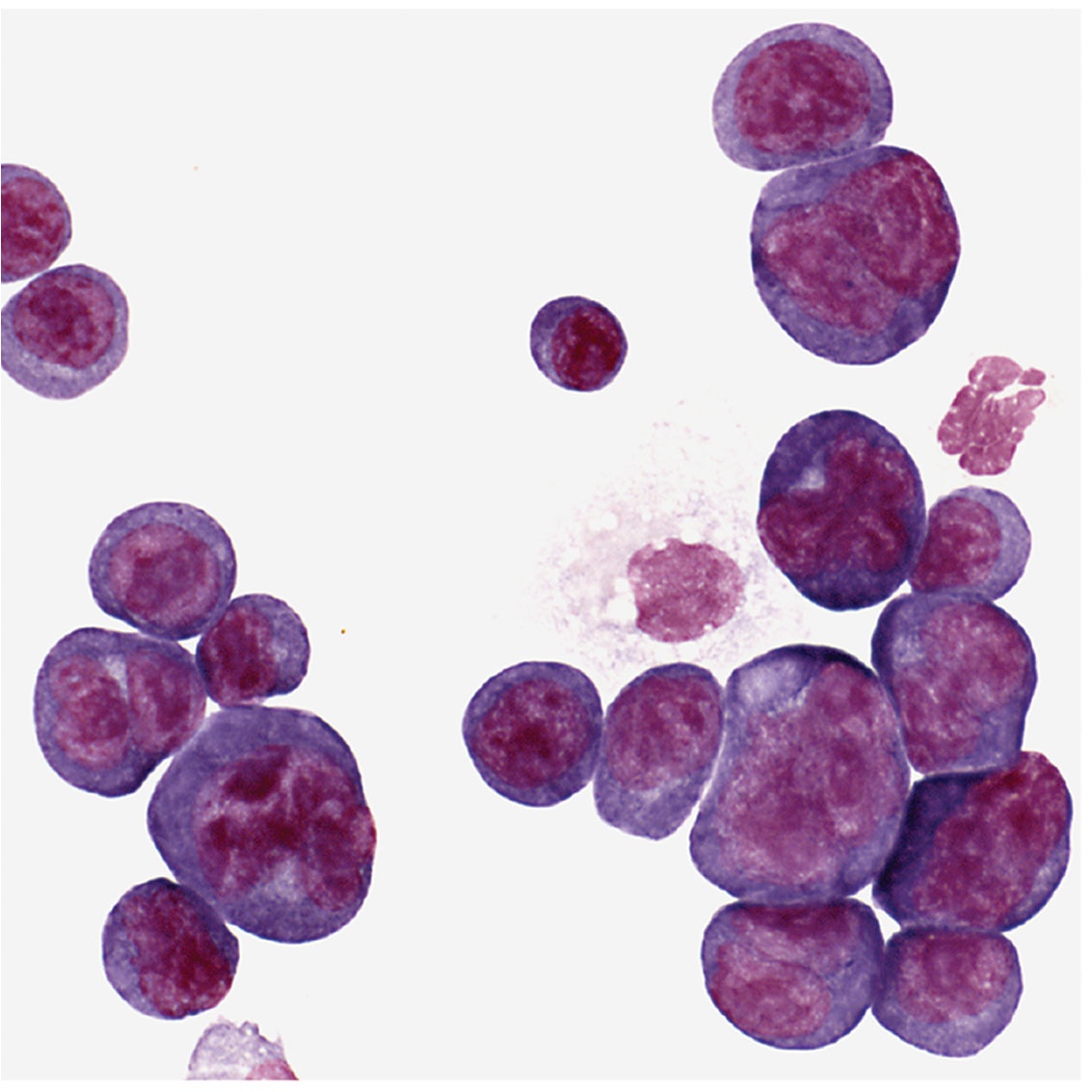
Potter was usually at his laboratory bench using this microscope. Shown are plasma cells with darkly stained nuclei. The clear spots next to the nucleus are perinuclear “hoffs” and are filled with newly synthesized protein, in this case, antibody.
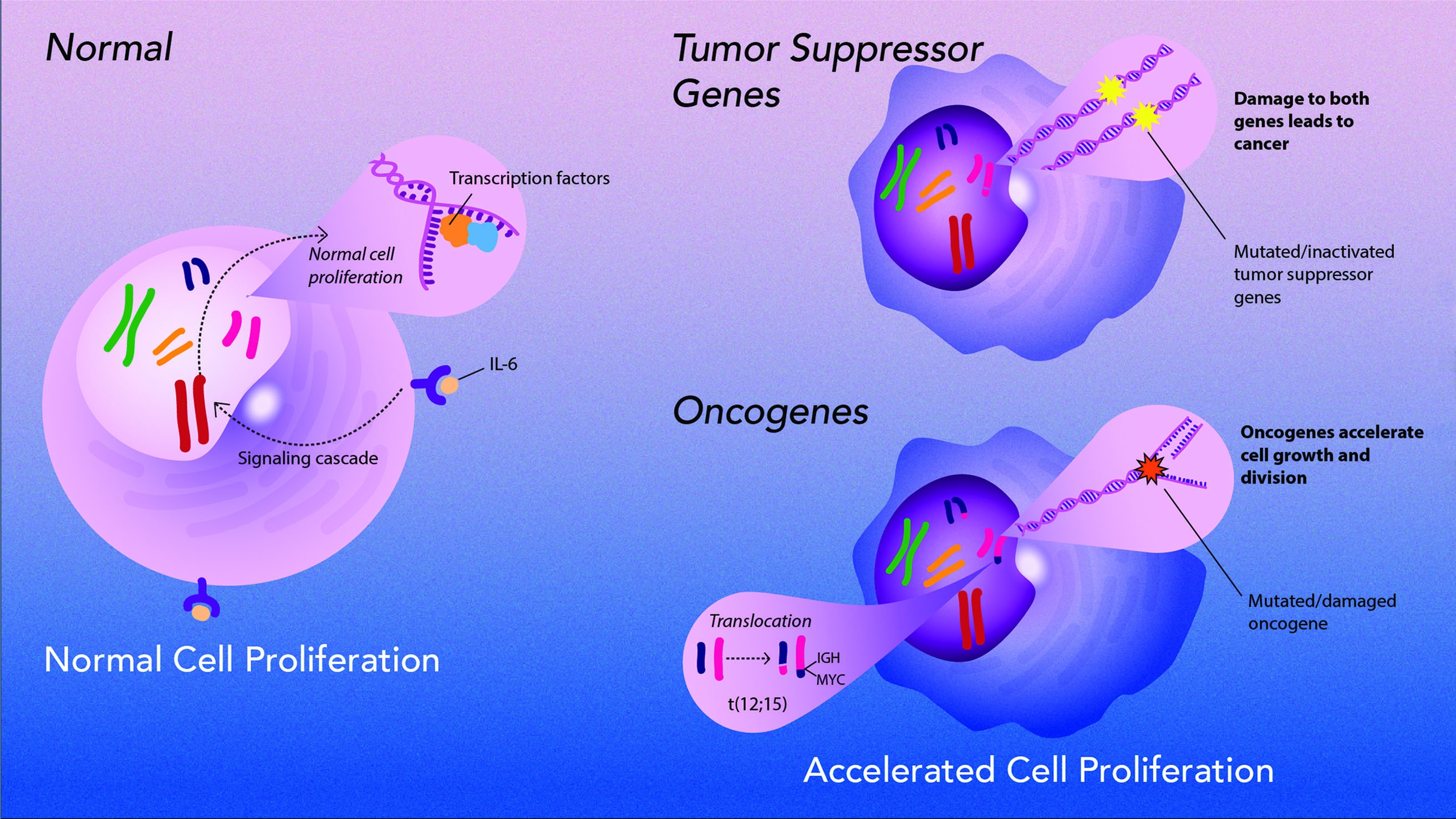
Retroviruses Oncogene Activation
Potter collaborated with Marshall Sklar and Wallace Rowe of the National Institute of Allergy and Infectious Diseases to see if viruses could affect the growth of Potter’s plasma cell tumors. Although viruses did not cause the tumors, they found that retroviruses containing oncogenes could accelerate tumor growth when injected into mice, and could even induce tumors in normally resistant mice. Grace Shen-Ong, who worked in Potter’s laboratory, and Michael Cole then found that plasma cells that develop tumors have chromosomal translocations involving genes for immunoglobulins and an oncogene called MYC. But Potter — working with J. Frederic Mushinski, Ken Marcu, Rex Risser, and Siegfried Janz — found that these translocations alone did not cause tumors. And retroviruses had to contain both MYC and another oncogene to induce plasma cell tumors.
Illustration designed by Sayeh Gorjifard, NCI, OD, CCT
Beverly Mock, Ph.D., Senior Investigator, Laboratory of Cancer Biology and Genetics, NCI/CCR NCI Collection. Photo by Bill Branson
Creating a New Technique
National Library of Medicine
Formal Recognition
“Dr. Potter's catalytic influence on the field of antibody research has extended far beyond his own laboratory through his generous sharing of materials, cell lines, information, and creative ideas.”
- —Lasker Foundation citation
The Ehrlich Prize
In 1983, the Paul Ehrlich Foundation awarded the Ehrlich Prize to Potter, Peter Doherty, and Rolf Zinkernagel for their immunological research. Potter had been elected to the National Academy of Sciences in 1981, an award he cherished because it was the result of the recognition of his peers.
Courtesy of Melissa Adde
The Lasker Medical Research Award
Michael Potter won the Lasker Award in 1984. Two of the other winners that year, Georges Kohler and Cesar Milstein, had just received the Nobel Prize for the development of monoclonal antibodies, which they could not have accomplished without Potter’s work or cell lines. Pictured: Henry Heimlich, who developed the Heimlich maneuver; Potter; Georges Kohler and Cesar Milstein on either side of Mary Lasker; and Paul Lauterbur, who helped develop magnetic resonance imaging. National Library of Medicine
Lasker Foundation press releases read as follows: 1984 Albert Lasker Medical Research Award Winners
Five distinguished scientists received the 1984 Albert Lasker Basic Medical Research Awards, presented by Mrs. Albert D. Lasker at the 39th Annual Luncheon, Friday, November 16, at the St. Regis-Sheraton Hotel in New York City.
Albert Lasker Medical Research Award
Mrs. Lasker congratulates the winners, from left, Dr. Henry J. Heimlich of Xavier University, Cincinnati, Ohio, for developing the Heimlich Maneuver which has saved thousands from death by choking; Dr. Michael Potter of the National Cancer Institute for his pioneering work with plasma cells; Dr. Georges J. F. Köhler of Basel, Switzerland, and Dr. César Milstein of Cambridge, England, for their revolutionary discovery of hybridoma technology; and Dr. Paul C. Lauterbur of Stony Brook, New York, for developing a remarkable new way to visualize the body’s internal organs without radiation. Drs. Milstein and Köhler are also winners of the 1984 Nobel Prize in medicine.
Michael Potter For his fundamental research in the genetics of immunoglobulin molecules and for paving the way for the development of hybridomas and monoclonal antibodies.
Dr. Potter’s work has centered on the plasma cell, a form of white blood cell, which acts as the antibody factory for the immune system. The first of his profoundly important discoveries came in 1956, when he found that adjuvants containing mineral oil could cause plasma cell malignancies, or plasmacytomas, in mice. These tumors can be transplanted among mice or grown indefinitely in the laboratory, and are the equivalent of multiple myeloma in humans. This experimental model of human disease has become a keystone of immunological research around the world. Dr. Potter identified immunoglobulins, which bind to specific antigens, making it possible to analyze the structure and the binding properties of antibodies.
1984 Abert Lasker basic medical research award presented by the Albert and Mary Lasker Foundation to Michael Potter, M.D. Chief, Laboratory of Genetics National Cancer Institute National Institutes of Health for his elegant studies of plasma cell tumors, leading to the development of monoclonal antibodies and enlarging our knowledge of carcinogenesis and the immune system.