Years of Change and Renewal 1969–1993
In two patients we had seen tumors shrink, and in one case disappear, after our immunotherapy. After all the deaths, after all the years in the lab, we had found something that worked. For the first time I believed — rather than hoped — immunotherapy not only could work, but would work.
- –Steven A. Rosenberg, M.D.
The Transformed Cell
During the era of President Richard M. Nixon, political turmoil engendered by the Vietnam conflict reverberated throughout the biomedical research world built by federal funding and NIH sponsorship in the previous decade. The Clinical Center had its antiwar demonstrations and counter-demonstrations, and civil rights issues led to a vigorous affirmative action program to widen the opportunities for minorities.
1
The war also brought demographic change within the hospital community. The end of the “doctor draft” in 1972 resulted in a steep falloff in Clinical Associate applications and jeopardized a critical source of new staff physicians. Normal volunteers were less often Mennonites and other conscientious objectors and increasingly were drawn from a national network of small colleges.
2
The greatest challenge the Clinical Center faced came directly from the Nixon administration. In the name of budgetary restraint and managerial efficiency, the administration sought to curtail research spending, reduce federal support for biomedical education, and to phase out the PHS hospital system. Congress, however, wanted to redirect spending away from the war effort. A collision course was set in 1971 and 1972 when broad majorities in both houses voted massive new outlays to conquer cancer, heart, and lung disease. The administration supported these initiatives but insisted that off-setting cuts be made in other health areas. As a result, the budgets of NIH categorical institutes other than Cancer and Heart, Lung, and Blood registered absolute declines in 1973.
3
A personnel ceiling remained in place for NIH as a whole, so that while NIH funding rose $946 million between 1968 and 1975, permanent staff lost 350 positions, and much of this burden fell on the Clinical Center. 4 Departments such as Clinical Pathology were able to contract out as much as half their work load, but others such as Nursing were forced to carry growing program commitments with fewer personnel. In 1972, its bleakest year, that department reported, “The quality of nursing care is obviously deteriorating, even though it is recognized that all personnel are doing their best.” 5 Demoralization was rife in scientific leadership as well. After three vetoes of the HEW budget and putative administration efforts to consolidate all the institutes into a single administrative structure, there was a real fear in the scientific community that the federal government might jettison commitments to support medical education, hospital construction, and basic research itself. 6
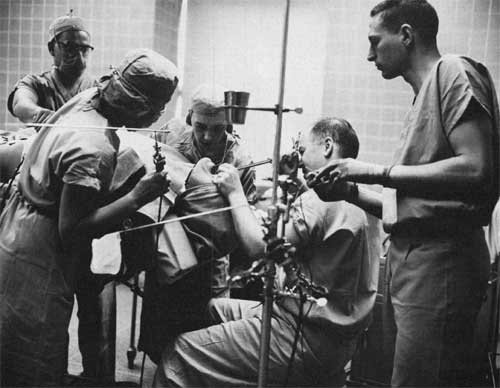
Dr. Andrew Morrow in surgery, inserting dye into patient's heart with a bronchoscope, a technique developed at the Clinical Center.
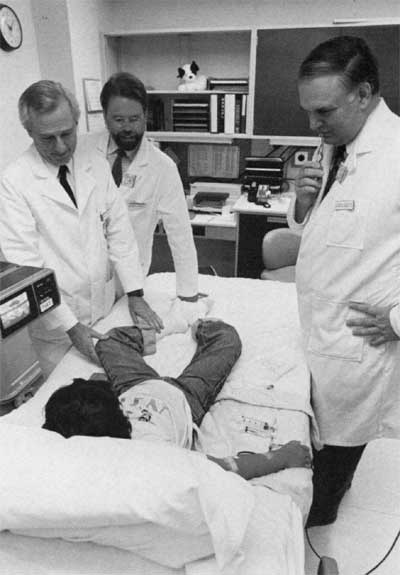
Dr. W. French Anderson (l.), Dr. Michael Blaese (r.), and Dr. Kenneth Culver (c.) attending the first patient in the ADA gene therapy program, September 1990. The patient is undergoing apheresis.
Reassessment and Renewal
For the Clinical Center, the task of renewal fell initially to Dr. Thomas C. Chalmers, a Harvard hospital director and gastroenterologist who was brought on as Masur’s successor in February 1970. Although Chalmers lacked an NIH background or PHS status, his initial efforts to upgrade clinical services were strongly supported by the new NIH director, Dr. Robert Q. Marston, who blocked administration efforts to assess Clinical Center research patients for insurance reimbursement.
7
Chalmers modernized clinical practice by establishing guidelines for transplant operations and preparing for computerization of medical records.
8
To restore morale among Clinical Associates, he transferred the responsibility of blood drawing to technicians and nurses, reinstituted patient-oriented clinical rounds, and pressed for formal residency training.
9
When the nursing staff shortage reached a crisis point in September 1972, Chalmers launched a drive to recruit 150 new nurses in eight months and to reorient the service in the long term toward acute care.
10
These goals were largely met by 1975, but Chalmers’ efforts to centralize clinical research oversight ran up against the increasing interpenetration of NIH administrators into hospital operations. The Medical Board, since 1969 responsible to the NIH Deputy Director for Science, allowed research oversight to devolve to the institutes. Rather than develop its own standards, the Board followed the lead of congressional mandates for the extramural program.
11
Dr. Chalmers’ most ambitious modernization initiative was his 1970 proposal for an “ambulatory research center” to be built over the main entrance on the north side of the original building. Extending the main building floors into this area would provide space to relieve overcrowding and new therapy centers for the burgeoning Outpatient Department. But the most radical feature of Chalmers' concept involved expanding employee health care programs and using NIH personnel “for innovative research in preventative medicine” and “for research in diagnostic programs.”
12
Congress appropriated planning funds through NCI at the end of 1971, and when these were reprogrammed Chalmers secured $3.5 million the following year for architectural services.
13
The administration blocked the funds’ release during 1973 while pressing again for insurance reimbursement from research patients. In January, Marston left the directorship, but his replacement, Dr. Robert S. Stone, a management professor at the Sloan School at MIT who had previously directed the New Mexico School of Medicine, also defended Clinical Center policy of not charging research patients.
14
As the Watergate investigation began to paralyze government in the fall of 1973, Dr. Chalmers quietly reduced the patient census and secured appointment as Dean of Mount Sinai School of Medicine in New York City in October.
15
Chalmers’ successor, Dr. Robert S. Gordon, Jr., was the first of four successive internal candidates to head the hospital after 1973. Clinical director for NIAMD since 1964 and Medical Board chairman for 1970, Gordon continued the work of reorganizing the hospital administration and upgrading clinical services after his appointment in January 1974. Convinced that a clinical research resurgence would revitalize the operating departments and attract higher quality staff physicians, Gordon teamed up with NIH Deputy Director for Science De Witt Stetten, Jr., to campaign for intramural funding for the clinical service departments through the National Institute for General Medical Science. A special congressional mandate could not be arranged, and several of the departments preferred to specialize in support services while allowing senior physicians to augment their salaries by contract work with the institutes.
16
Gordon also tried to give the clinical directors a stronger role in ensuring the quality of patient care and providing research oversight.
17
While these traditional gambits of centralized clinician responsibility and categorically defined basic research were now less availing, ever more sophisticated clinical applications emerged at this point as a clear trend. A contract was awarded in March 1975 for design of the addition to the Clinical Center, planned to accommodate 200,000 outpatient visits annually. Another was awarded the following June for the first hospital-wide computer system to process patient data, research information, and routine administration.
18
Growth and Expansion
The turning point in the renewal process came in the summer of 1975, as Dr. Donald Fredrickson took over as NIH director and Dr. Gordon retired from the PHS and announced plans to take an academic appointment. Determined to reassert and redefine the NIH’s mission in the face of rising public demands on biomedicine and increasing fragmentation within disease categories, Fredrickson brought a new activism and vision to the political process, a willingness to overcome deficiencies and to engage state-of-the-art problems with the expectation of optimal results.
19
In short order Fredrickson secured a $90 million construction appropriation for the Ambulatory Care Research Facility (ACRF), proposed a new agency focus on clinical trials, and called a three-day retreat at Easton, Md., to review clinical operations, address deficiencies, and assess emergent clinical research needs. Out of this January 1976 conference came decisions to create an intensive care unit and to centralize quality assurance under a strengthened Medical Board.
20
In July, hospital leadership passed to Dr. Mortimer B. Lipsett, an endocrinologist with previous service as branch chief in NCI and NICHD. Lipsett simplified research review and made it a Clinical Center responsibility by setting up institute panels monitored by the CC’s Office of Planning and Policy Development. Lipsett also renewed Gordon’s effort to obtain a separate congressional appropriation for the hospital. He formalized a mission statement for the Clinical Center that placed patient care ahead of research requirements and detailed for the first time ancillary responsibilities in the areas of bioethics, information dissemination, and in-house diagnostics and patient care research.
21
From May 1977, when excavation began for the ACRF, until January 1982, when the first patients were moved in, construction was a constant feature of hospital life. Numerous areas of the old building underwent renovation, and program modernization became endemic for a wide variety of activities. Lipsett registered efficiency gains by raising bed occupancy from 65% to 75% and by opening new services, particularly Critical Care Medicine. However the serving Clinical Associates still considered the Clinical Center “not a full service hospital.” 22 Adverse political influences continued, particularly the recurrent demand to bill research patients and the reimposition of personnel ceilings in 1979, which threatened 250 positions out of 1,573. 23 Inflation prevented planned expansion of clinical trials in 1980 and 1981, but research conducted by the operating departments showed new promise. Investigators from the Blood Bank and NIAID identified “non-A, non-B hepatitis” as the source of 80% of transfusion-related cases. 24 This allowed comprehensive screening of blood and blood products, thus dramatically increasing safety of transfusion. Another significant innovation was positron emission tomography (PET), which the Nuclear Medicine Department began using to do brain scans in 1979 and to support early research into Alzheimer's disease.
With the resumption of institutional growth and budgetary expansion in the Fredrickson era, prospects again seemed hopeful for new advances in clinical research. In 1982 three intramural researchers shared Lasker Foundation Awards for molecular-level discoveries with important therapeutic effects: Dr. Robert C. Gallo (NCI) for work leading to isolation of the human retrovirus; Dr. Elizabeth F. Neufeld (NIADDK) for identifying the enzyme defect causing mucopolysaccharide storage disorders; and Dr. Roscoe O. Brady (NINCDS) for demonstrating the metabolic causes of lipid storage diseases.
25
In 1983 a comprehensive AIDS research program was announced, featuring 25 intramural investigators and focused on Critical Care Medicine patients. The following year Dr. Steven A. Rosenberg began Phase I trials in immunotherapy, and Dr. W. French Anderson initiated gene therapy experiments, which would lead, by decade’s end, to a proliferation of genetic research and prospective cures for many metastatic cancers. 26 Also in 1984, Gallo’s confirmation that the retrovirus HIV causes AIDS placed Clinical Center laboratories and researchers at the vital center of the emerging field of molecular medicine.
The challenge of reorienting hospital activities fell to Dr. John L. Decker, NIADDK clinical director, who was appointed Clinical Center director in August 1983. Faced with dramatic growth in outpatient services and Reagan administration actions to freeze staff positions and require payments from patients, Dr. Decker and his staff decided in January 1985 to contract out Anesthesiology and Surgical Services in order to increase outpatient staffing. Representatives Natcher and Dingell of the Appropriations Health Subcommittee prevented the patient-payments provision from becoming law in 1985, and in the following year Congress began to increase steeply NIH’s budget for AIDS research.
Further readjustments were finalized at a second administrative retreat at Easton in January 1988. The hospital would continue to support “modest growth” in emergent areas such as bone marrow transplantation, and clinical expenses would be more closely regulated by putting institute funding of central services on a more flexible, patient/day basis. When Dr. Decker retired in June 1990, his successor, Dr. Saul Rosen, focused hospital management on quality assurance and the restoration of clinician confidence in patient care activities.
27
A New Research Revolution: Molecular Medicine
Between 1983 and 1993, the development of recombinant DNA technologies has stimulated a new research revolution, and the Clinical Center continued to thrive. A growing stream of clinical research advances since 1989 has brought renewed distinction to its laboratories and added new mandates to its mission. In a series of four gene therapy protocols, begun in September 1990, NCI and NHLBI researchers demonstrated the cancer-killing potential of tumor-infiltrating leukocytes and through gene therapy restored the immune system in two young girls suffering from ADA deficiency, a rare genetic disease.
28
Molecular medicine advances are now being reported in a widening circle of clinical fields. NHLBI researchers have used gene therapy techniques to transfer normal genes into airway cells of rats to correct the devastating symptoms of cystic fibrosis, and human trials are currently underway. The Clinical Center has also been a nationwide focus for AIDS research. NIAID and NCI researchers discovered zidovudine (AZT) and determined its efficacy for pediatric AIDS, and they have also played a leading role in establishing a national trials program. In fiscal year 1990, 22% of NIH intramural clinical trials funding was allocated to AIDS programs.
29
In addition, the Nursing Department has opened day hospitals and conducts a growing array of clinical research projects in partnership with clinical services of the categorical institutes. Long-standing disparities in nursing salaries have been corrected, and a full staff complement is now a permanent feature of hospital operations.
The promise of prospective cures, which is endemic to this research, energizes scientists and clinicians today no less than in previous decades. The conquest of infectious disease announced by Surgeon General William H. Stewart in 1969 was premature, and the nation now faces a pandemic of HIV infection and the recurrence of old plagues, such as tuberculosis.
30
But these challenges to scientific creativity are the surest signs that the Clinical Center will continue to renew itself and to widen the perimeters of human health.
References
- Minutes, Clinical Center staff meetings: May 6, 1969; July 15, 1969; November 18, 1969; April 20, 1971; box 3, RG 443. ↩
- Minutes, Clinical Directors special meeting, February 25, 1974; minutes, Medical Board meeting, October 27, 1970. ↩
- Richard A. Rettig, Cancer Crusade: The Story of the National Cancer Act of 1971, Princeton: Princeton University Press, 1977, pp. 30-35; Science, 183: 1325-26, December 28, 1973. ↩
- NIH Program Review, 1975, box 8, folder, “Clinical Center Reorganization, 1974-1975,” RG443. ↩
- Memorandum, Director, Clinical Center to Director, NIH, 11/20/73, “Review of Institute Contracts. . .“ Clinical Directors meeting file, box 3, RG 443; Annual Report of Program Activities, Clinical Center, 1972, Nr 6. ↩
- Science, 179: 546-47, February 9, 1973; 180: 843-44, May 25, 1973; 182: 460-61, September 23, 1973; 183: 1325-26, December 28, 1973; Burroughs Mider, “The Federal Impact on Biomedical Research,” in John Z. Bowers and Elizabeth Purcell, eds., Advances in American Medicine at the Bicentennial, vol. 2, New York: Josiah Macy, Jr., Foundation, 1976, pp. 861-64. ↩
- Marston to Assistant Secretary for Health, September 5, 1969, attached to minutes, September 15, 1969, Clinical Directors meeting, box 3, RG 443. ↩
- Minutes, Medical Board meetings, March 23, 1971, p. 3, and May 25, 1971, p. 5; draft, proposed memorandum, Medical Record Committee, 1971, Medical Board file. ↩
- Memorandum, E. Lawrence, Clinical Center Executive Officer, “Meeting with NCI Clinical Associates..." January 1, 1971, box 7, miscellaneous minutes folder; Chalmers to Clinical Directors, September 9, 1970, “Clinical Center Rounds...,” box 3, Clinical Directors minutes file; minutes, Department Heads meeting, February 16, 1971, same file. ↩
- Memorandum, Chalmers to Clinical Directors, September 29, 1972, “Nursing Shortage... ,“ Clinical Directors meeting file. ↩
- Minutes, Medical Board meetings: February 10, 1970; April 9, 1970; March 13, 1973. ↩
- Chalmers to Marston, July 27, 1971, minutes file, Clinical Directors meetings, box 3, RG 443. ↩
- Minutes, Department Heads meeting, February 15, 1972, box 3; 1975 Program Review, p. 5-2-2, box 8, RG 443. ↩
- Science, 180: 1258-61, June 22, 1973; minutes, Clinical Directors meeting, November 5, 1973; Memorandum, Stone to Weinberger, November 21, 1973, same file. ↩
- Science, 180: 1157, June 15, 1973; minutes, Clinical Directors meeting, November 4, 1973, p. 2. ↩
- Memoranda, Stetten to Stone, “Intramural Program for NIGMS... ,“ February 7, 1974; J. Doppman to J. Block, February 19,1974; Clinical Pathology survey, same date; Gordon to Stone, April 23, 1974, all in box 8, folder “Clinical Center Reorganization, 1974-1975,” RG 443. ↩
- Minutes, Medical Board meeting, March 12, 1974, and Clinical Directors special meeting, June 30, 1975. ↩
- Annual Report of Program Activities, Clinical Center, FY 1975, Dir-1. ↩
- Agenda meeting of Intercouncil Representatives, July 16, 1975, Fredrickson file, minutes, 1975, folder 3, box 2, RG 443. ↩
- Minutes, Clinical Center Department Heads meeting, August 13, 1975; draft, “Review of Activities,” Director, NIH, July 16, 1976, Fredrickson file, minutes, 1976; minutes, Medical Board meetings: March 9, 1976; March 16, 1976; April 15, 1976. ↩
- Annual Report of Program Activities, Clinical Center, 1977, OD-1 ,3,1 0; minutes, Medical Board meetings: August 3, 1977; September 7, 1977. ↩
- Minutes, Medical Board meetings: November 2, 1976; December 6, 1976; December 5, 1978; Annual Report of Program Activities, Clinical Center, 1977, OD-2; Annual Report..., 1979, OD-3. ↩
- Minutes, Medical Board meetings: October 4, 1977; September 7, 1979. ↩
- Briefing paper for HEW by Director, NIH, July 17, 1979, pp. 5-6, Fredrickson files, minutes, 1979, folder 1, box 3, RG 443; Annual Report of Program Activities, Clinical Center, 1979, OD-8. ↩
- NIH Record, 34, December 7, 1982. ↩
- NIH Record, 34, March 29, 1983; Steven A. Rosenberg, The Transformed Cell: Unlocking the Mysteries of Cancer, New York: G.P. Putnam’s Sons, 1992, pp. 179, 201, 265. ↩
- Minutes, Medical Board meeting, January 15, 1985; memorandum, Surgical Administrative Committee, January 14, 1985, same file; Report to the Medical Board, Annual Report, Clinical Center, FY 1990, pp. 55-57. ↩
- W. French Anderson, “Human Gene Therapy,” Science, 256: 808-13 (May 8, 1992). ↩
- Institute of Medicine, Report of a Study: The AIDS Research Program of the National Institutes of Health, Washington, DC: National Academy Press, 1991, p. 85. ↩
- Robin M. Henig, A Dancing Matrix: Voyages Along the Viral Frontier, New York: Alfred A Knopf, 1993, xi-xiii. ↩