Protein Structure and Synthesis: The Road to the Nobel Prize
How Do Proteins Fold? The Thermodynamic Hypothesis
Proteins fold into specific shapes to do their work. But how this happens was a major scientific mystery. Anfinsen wanted to know if positioning the right number of the right amino acids in the right sequence was enough to form a folded protein. For this work he used ribonuclease (RNase) from cows—obtained, interestingly enough, from a slaughterhouse.
This drawing from Anfinsen’s papers shows folded RNase on the left and denatured (unfolded) RNase on the right. The arrows face both ways to indicate that proteins can fold or unfold in a reversible process.
Eventually, Anfinsen’s conclusions became known as the Thermodynamic Hypothesis: the three-dimensional form of a protein is determined by the protein’s amino acid sequence and the environmental conditions in which the folding occurs.
Anfinsen unfolded RNase by dissolving its disulphide bonds, the bonds in its outer structures, and used special solvents, leaving the original amino acid chain intact. When he removed these chemicals, the protein automatically refolded into its active shape. This experiment demonstrated that the three-dimensional shape of a protein was determined by its amino acid sequence, confirming his hypothesis.Image from The Molecular Basis of Evolution.
Anfinsen (right) contemplates a three-dimensional model of RNase.National Library of Medicine
Can We Make Proteins?
In 1962, Christian Anfinsen left the NIH, after 12 years in Bethesda, for Harvard Medical School, but returned months later to the National Institute of Arthritis and Metabolic Diseases (now the National Institute of Diabetes, Digestive, and Kidney Diseases). As chief of its Laboratory of Chemical Biology, Anfinsen began researching the nuclease protein of the bacteria Staphylococcal aureus. His goal was to discover the protein’s amino acid structure and then synthesize it himself in the laboratory.
By 1966, Anfinsen and his colleagues had isolated Staphylococcal nuclease. Just as in the beef RNase, they found that the unfolded chain could refold into the correct shape. A year later, they had the sequence of the 149 units in the amino acid chain.
Finding the Right Technologies
Along the way, Anfinsen tried many purification methods and helped develop the powerful affinity chromatography technique. A molecule called a “ligand” that binds to a specific receptor is anchored to solid support. When a solution flows over it, the ligand binds to the specific molecule that has an “affinity” or natural bond with it. After the solution is washed away, the now purified molecule is stripped from its support.
Amazing to think that so simple an apparatus could yield such significant contributions to the understanding of protein structure. Columns like this are seen in many photos of Anfinsen’s lab equipment.
Learn more on the artifacts page: Pharmacia Fine Chemicals Affinity Chromatography Tube
From the permanent collection of the NIH Stetten Museum, courtesy of Dr. Christian Anfinsen
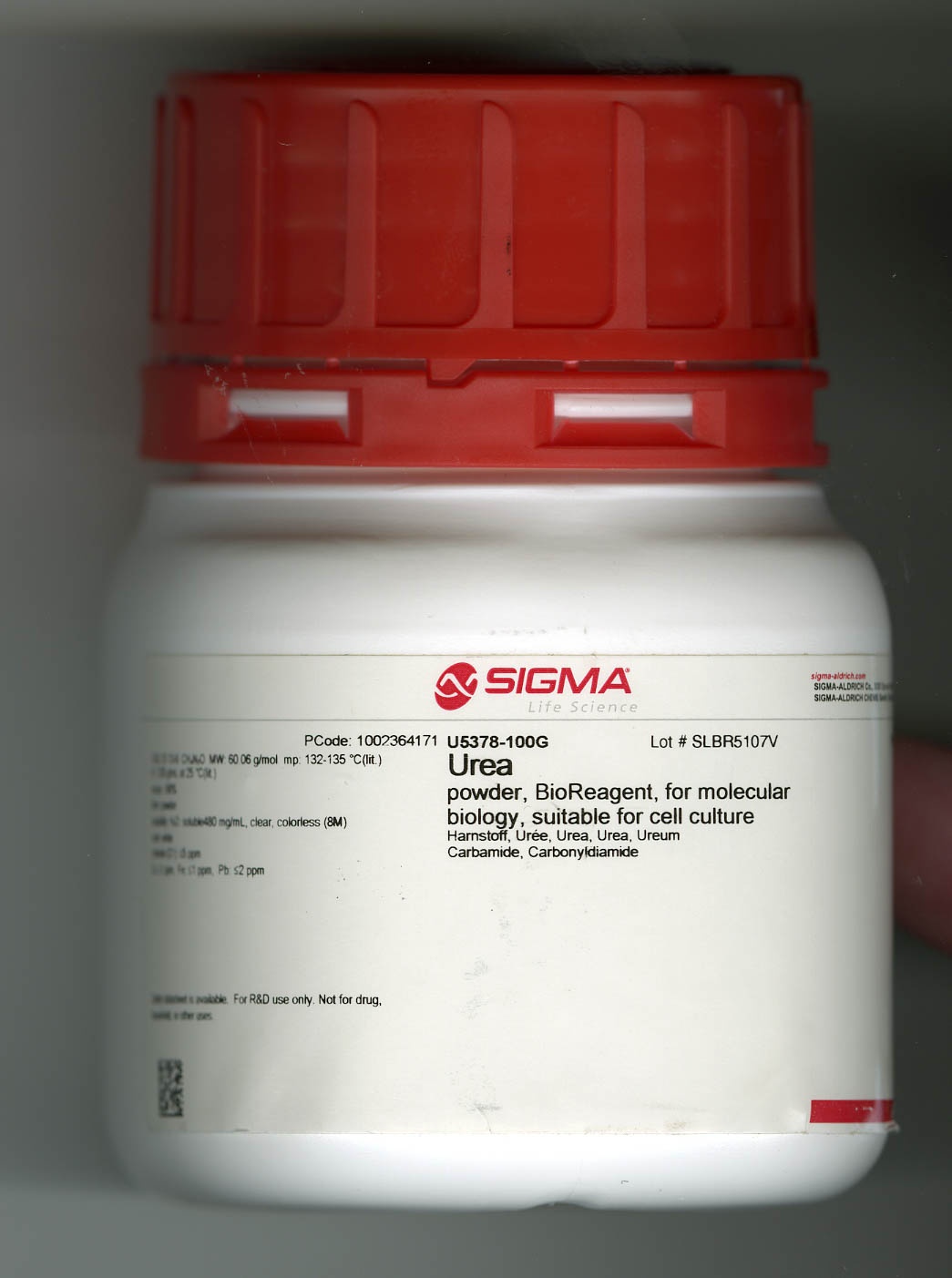
RNase B from bovine pancreas and urea. RNase B from bovine pancreas is now readily available to researchers.On loan from MilliporeSigma
Solid phase peptide synthesis, which bound a peptide to a support and washed any unreacted reagents away, was a new concept in 1959. Anfinsen tried this method, but it didn’t work well for large proteins.From the permanent collection of the NIH Stetten Museum, courtesy of Dr. Christian Anfinsen
In the lab with Sephedex column for affinity chromatography.U.S. Library of Medicine, courtesy of Libby Anfinsen
The Nobel Prize
Underneath this suave massive exterior, I’m shivering like a bowl of jelly.
- —as reported in the NIH Record, November 7, 1972
Dr. Anfinsen’s work on ribonuclease (RNase), which demonstrated that the information required to fold a protein into its particular three-dimensional active state came from its sequence of amino acids, was recognized with the Nobel Prize in Chemistry in 1972. He shared the prize with Stanford Moore and William H. Stein of Rockefeller University. This knowledge led several groups to be able to synthesize RNase, which was the first time that an enzyme had been artificially created from chemicals in the laboratory.
Anfinsen recreates for the NIH Record photographer how he was working in his laboratory when he received a call. “It was a reporter with the news that the researcher was to share the Nobel Prize in chemistry.” —NIH Record, November 7, 1972 National Library of Medicine
© The Nobel Foundation
His Royal Highness the Crown Prince Carl Gustaf of Sweden (left) presents Dr. Christian Anderson the 1972 Nobel Prize in Chemistry. National Library of Medicine