...
| Div | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
|
Creating New Drugs by Modifying the Molecule
...
| Div | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
|
...
| Div | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
|
Learning to Make Opiates Without Flowers
| Div | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| |||||||||
| Div | |||||||||
| |||||||||
| Div | |||||||||
| |||||||||
| Div | |||||||||
|
...
|
Photography Credits
| Note |
|---|
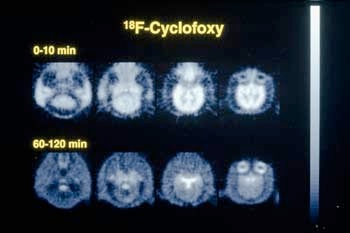
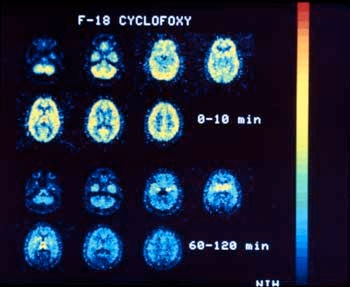
Please Note: Photographs are courtesy of Dr. Kenner Rice, Laboratory of Medicinal Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases. |
All images, text, transcription, and/or recordings reproduced in these exhibits and galleries may be protected by copyright. Users who wish to reproduce materials for other than private use should make an additional effort to determine ownership and, if restricted, seek permission before reproducing them.
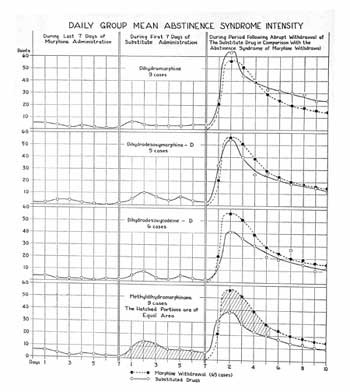
Metopan Diagram. C.K. Himmelsbach. Journal of Pharmacology and Experimental Therapeutics, Vol. 67, No. 2, October 1939, pp. 239-249.