Introduction
Humans have constantly searched for effective and safe relief from pain. One pain reliever, used from antiquity, has been opium derived from poppy plants. In the nineteenth century, the pain killers morphine, codeine, and thebaine were isolated from opium. Morphine and codeine quickly became important medicines for treating pain, cough, and diarrhea. This exhibit tells how 20th century researchers at the National Institutes of Health created new opiate drugs and developed a synthetic source for morphine and codeine -- and why.
The Drug Design and Synthesis Section of the Laboratory of Medicinal Chemistry (LMC) is part of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Its mission is to identify the structure and function of chemical messenger systems in the brain and to determine how drugs, especially opium, affect the brain on a molecular level.
The Drug Design and Synthesis Section of the Laboratory of Medicinal Chemistry (LMC) group
Seated in the first row Ross Johnson, Marina Mattson, Kenner Rice, Margaret Aein, Arthur Jacobson. Standing in the middle Row are: Dorota Mateka, Neil Grayson, Guerdy Toussaint, Lilian Radesca, Celia Dominguez, Zi-Qiang Gu, Brian de Costa. Standing in the third and final row are: Dragona Tadic, Wanda Williams, Xiao-Shu He, Dorothea Thomasson, Jan Linders, Wayne Bowen, John Glowa, Bertold Vilner. (December 1991).
From Flower to Medicine
Sap from the poppy Papover somniferum has been used for thousands of years to relieve pain and treat symptoms of disease. Poppies are grown only in certain areas of the world, and supply the entire world’s supply of opiate pain killers.
The poppy Papover Somniferum
Raw opium oozes from a lanced poppy seed pod grown by licensed farmers in Turkey and Iran.
A brick of pressed raw opium ready to be transported for processing.
Creating New Drugs by Modifying the Molecule
When scientists first began looking at drugs derived from opium ("opiates"), they were interested in identifying drugs that would be good painkillers, but would not lead to patients' becoming addicted to the drug -- a problem with codeine and morphine.
In 1929, Dr. Lyndon F. Small established the Drug Addiction Laboratory, the ancestor of today's LMC. Seeking to create painkillers which would not cause addiction, Small studied and modified the morphine molecule and related opium products. This work was based on the assumption that the drug's effects were directly related to its molecular structure. Small's assumption proved correct. Metopon, a potent opiate painkiller, was among his most notable contributions. With metopon, partial separation of the painkilling effects from the undesired addictive property was at last achieved. This finding was the underpinning of much research that continues today into a total separation of painkilling and addictive effects.
Dr. Small prepared hundreds of potential opiate drugs for pharmacological evaluation, keeping them in cigar boxes. Small's samples have been used by many investigators to identify opiate products.
Public Health Service physician Clifton K. Himmelsbach
Dr. Nathan Eddy evaluated Small's compounds for their painkilling effectiveness and potential to be addictive, and Dr. Clifton Himmelsbach conducted the first clinical tests with these drugs in human subjects. Early studies on metopon showed it produced a less severe addiction than morphine and other opiates. This finding encouraged scientists to hope that the addictive side effect could be further separated from the painkilling action.
Dr. Everette May and Dr. Eddy worked to develop opiates that relieved pain without the potential for abuse and to discover synthetic substitutes for opiates--called opioids. Based on May's work on benzomorphans, the drug pentazocine was introduced in the 1960s. Pentazocine was the first drug used in clinical practice as a painkiller which combined the pain-relieving effects of morphine with the effects of opiate antidote. Such drugs are called mixed agonists-antagonists because they produce some opiate effects while blocking the opiate effects of other drugs. But pentazocine was not as effective as morphine for severe pain, and produced hallucinations at higher doses.
Medicinal chemist Everette May and pharmacologist Nathan B. Eddy
The drug buprenorphine was developed using the agonist-antagonist construct. It is a promising drug for the detoxification of heroin addicts because it produces less intense withdrawal symptoms, is relatively non-toxic, and can be used by outpatients. It is also being studied as a therapy for individuals dependent on cocaine.
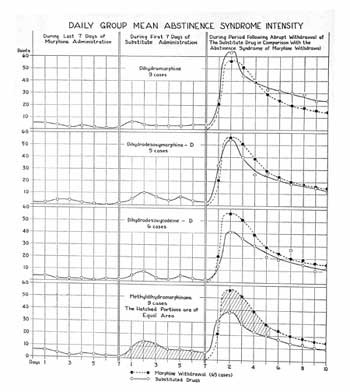
The bottom shows that metopan (methyldihydromorphinone) fails to block morphine withdrawal symptoms completely (center) and that its own withdrawal syndrome is milder than that produced by morphine (right)
C.K. Himmelsbach. Journal of Pharmacology and Experimental Therapeutics, Vol. 67, No. 2, October 1939, pp. 239-249.
Using Opiates to Learn About the Brain
The discovery that the brain produces its own chemicals which act similarly to opiates opened avenues for learning how the brain transmits signals and manages thought, feeling, and action. It opened new ways to study diseases as well.
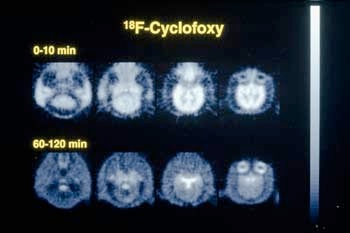
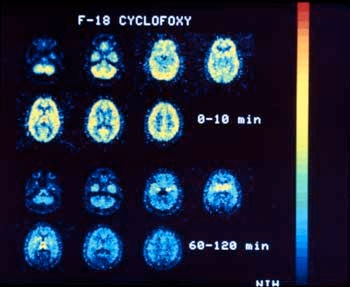
In 1984, using their opium-derived narcotic antagonist called cyclofoxy, Dr. Kenner Rice's group at LMC and their NIH associates reported the first definitive image of opiate receptors in a living primate brain. They are now using this drug and other research tools to investigate central nervous system disorders, the regulation of the immune system, and possible new treatments for drug dependence.
Besides being painkillers, certain narcotic drugs and antagonists (drugs which block the actions of other drugs by combining with and blocking receptors) are used to study opiate receptor sites in normal humans. Parallel studies in patients with schizophrenia, bulimia, sexual dysfunction, and opiate and cocaine addition will provide insight into the role of opioid receptors in these disorders. A PET (positron emission tomography) scan shows cyclofoxy binding to receptors in the caudate nucleus, thalamus and other areas of the brain (red to orange areas).
Light portions in the center of the brain images show that cyclofoxy is binding to opiate receptors in a baboon brain
Learning to Make Opiates Without Flowers
In the early 1970s, a severe opium shortage was created by increased demand, poor poppy crops, a Turkish ban on poppy cultivation, and the Soviet Union's entry as a buyer of opium on the world market. In 1973, 45% of the United States' strategic stockpile of opium had to be released for domestic use. Finding a way to manufacture totally synthetic opium products from readily available materials became imperative.
On January 22, 1979, Dr. Kenner Rice of the LMC discovered the critical chemical reaction enabling large-scale production of totally synthetic morphine, codeine, and thebaine, the three basic raw materials in opium. As shown in his laboratory journal, the initial sign of success was the identical chromatographic behavior of Dr. Rice's product and an authentic sample from opium. Dr. Rice's method, now internationally known as the NIH Total Opiate Synthesis, is still the only practical process available for making large quantities of opium products from synthetic materials, guaranteeing reliable supplies of opiate pain relievers.
A Page from Dr. Rice's Laboratory Journal
Photography Credits
Please Note: Photographs are courtesy of Dr. Kenner Rice, Laboratory of Medicinal Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases.
All images, text, transcription, and/or recordings reproduced in these exhibits and galleries may be protected by copyright. Users who wish to reproduce materials for other than private use should make an additional effort to determine ownership and, if restricted, seek permission before reproducing them.
Metopan Diagram. C.K. Himmelsbach. Journal of Pharmacology and Experimental Therapeutics, Vol. 67, No. 2, October 1939, pp. 239-249.